BASIC PHOTOIMMUNOLOGY
Faith M. Strickland
Department of Internal Medicine, Rheumatology Division
The University of Michigan, Ann Arbor, MI 48109-2200
fmstrick@med.umich.edu
Introduction
Photoimmunology is the study of the effects of light on the immune system. Light, technically known as nonionizing electromagnetic radiation, is divided into different regions of the electromagnetic spectrum, UV-C (<280 nm), UV-B (280-320 nm), UV-A (320-400 nm) based on physiological effects by photons in those regions (for abbreviations used here, see Table 1) . The reader is referred to the basic Ultraviolet Radiation Photobiology and Photophysics modules for further explanation on the properties of light and its molecular targets in tissues. The concept that 'light', which is a part of the external environment, can affect the immune response, whose organs and components are internal, arose from experiments by Margaret Kripke and collaborators in the Mid-1970's aimed at discovering a role for the immune response in rejecting UV radiation-induced skin cancer cells (reviewed by Schwatz, 2008). Their work demonstrated that UV radiation exerted suppressive effects on immune responses to UV-induced skin cancer cells, and that the inhibition of tumor rejection could be adoptively transferred by T lymphocytes in an antigen-specific manner. Subsequent work by many laboratories revealed details on how UV radiation induced the suppression of immune responses (also known as 'immune tolerance'). This suppression was observed not only for skin cancers, but also infectious agents and chemical antigens, and involved a role for DNA repair as well as a complex interaction between the immune system and the skin. Together, these studies gave rise to the field of Photoimmunology. This module focuses on the effects of the ultraviolet portion of the electromagnetic spectrum on the immune response, and how it affects the cells and components of the immune system to alter the balance and functions of immune cells to infectious agents, chemicals, and skin cancer.
Immune Response
The immune response is mediated by the complex interaction of bone marrow-derived white blood cells, whose function is to distinguish what is host (self) from what is externally derived (non-self), and to eliminate what does not belong to the host. The balance of effector and repressor mechanisms is an essential feature of the immune system. An imbalance of effector and repressor mechanisms leads to a failure to respond (e.g., congenital or acquired immune deficiency diseases) or over-reactivity (e.g., autoimmune diseases). This failure to distinguish the host from non-self can result in the destruction of host tissues and even death.
The immune response is broadly divided into innate and adaptive arms. Innate, or non-specific responses recognize non-self via biochemically based recognition mechanisms, and is mediated by macrophages, natural killer (NK) cells, mast cells, granulocytes, and fragments from a series of enzymatically activated proteins called 'complement'. Cells of the innate immune systems secrete a variety of toxic substances including oxygen free radicals and enzymes aimed at killing invading organisms, and recruiting other members of the innate and adaptive immune system to the site of infection. Innate immune responses begin to act within minutes to attack, and eliminate infectious organisms, parasites, and foreign objects (for example, rose thorns). Macrophages (macro = large; phage = eat) are large cells that engulf and digest organisms, or if that is not possible (e.g., carbon particles), they retain the object within their cytoplasm, and effectively remove it from contact with tissue. Dendritic antigen presenting cells are a related to macrophages, but take up small substances such as proteins, peptides, viruses. Dendritic cells are the professional antigen presenting cells (APC) that process the agents and present them to T lymphocytes in the context of appropriate recognition and costimulatory molecules required to induce a T cell-mediated immune response (discussed in more detail below). In the skin, APC's called Langerhans cells form a network of cells interspersed between keratinocytes in the epidermis. Langerhans cells act as a 'surveillance system', detecting and transmitting foreign antigens for recognition by the adaptive immune system.
The adaptive immune response is much slower to develop, days to weeks, and is a second line defense mechanism following innate immunity. The adaptive immune response is mediated by a cellular arm consisting of lymphocytes, and a soluble arm made up of antibody and proteins called cytokines. The lymphocyte fraction is made up of B cells, plasma cells, and T cells. T cells are further divided into subsets such as CD4+ helper, CD8+ cytotoxic, NKT cells, and regulatory (previously known as suppressor) T cells that can be either CD4+ or CD8+ (Delves et al., 2006). T cells bind and respond to antigen when it is presented by dendritic cells in the context of major histocompatibility and co-stimulatory molecules. A lack of dendritic cells (as occurs in UV-irradiated skin), and altered DC functions can result in a failure of T cells to respond to antigen. B cells bind antigen directly through their antigen receptor (immunoglobulin, Ig), which triggers B cells to produce and secrete large amounts of soluble IgM, and with help from T cell-produced factors, IgG, IgA and IgE subclasses of antibody.
Photocarcinogenesis and the Skin Immune Response
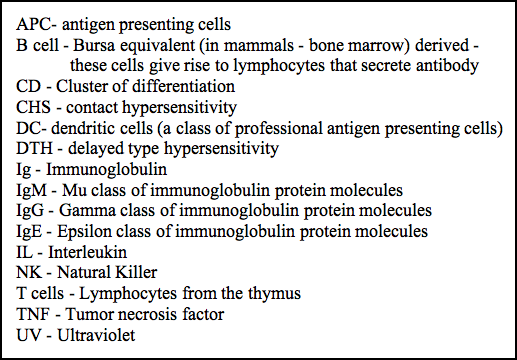
Photoimmunology arose from Photocarcinogenesis in the mid-1970's when Margaret Kripke and her colleagues discovered that UVB (280-320 nm) radiation was not only carcinogenic but also contributed to the growth of newly transformed skin tumors by suppressing the immune response that would otherwise have rejected the tumor (Kripke 1974). Her model is illustrated in Figure 1.
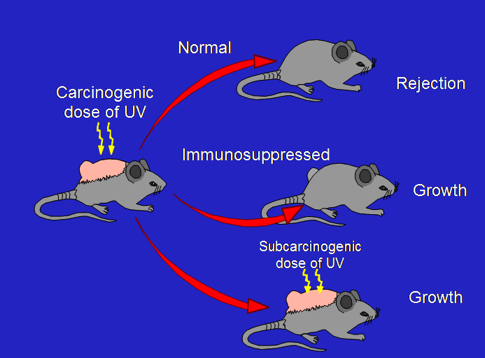
Figure 1. The immune response plays a role in skin tumor growth (Kripke, 1974).
Chronic exposure of the skin of humans and laboratory animals causes mutations in the DNA of skin cells, eventually leading to the transformation of some of these cells to cancer. Skin tumors induced in mice by UV radiation were highly antigenic, and would be rejected when transplanted into genetically identical mice that had not been exposed to UV. These same tumors would grow when transplanted into immunosuppressed mice, showing that the immune system could readily recognize and reject UV-induced skin cancers. What was surprising, however, was that when these same tumors were transplanted into mice that were treated with a subcarcinogenic dose of UV radiation, transplanted UV-induced tumors would grow while skin tumors induced by chemical carcinogens would still be rejected. These results indicated that UV radiation not only caused skin tumors, but prevented their rejection by the immune system. Furthermore, this immunosuppression was not general but was specific for antigens present on UV-induced tumors. Tumor-specific immune suppression could be transferred by injecting T cells from UV-irradiated mice into untreated recipients and transplanting tumors, which subsequently grew in these recipients but not in mice that received normal T cells (reviewed by Schwarz, 2008). Thus, the immune response could recognize and reject UV-induced skin tumors, but was prevented from doing so by mechanisms that are still being elucidated. Some of these mechanisms are discussed below.
Although the first evidence that radiation in the UV wavebands can affect the immune response came from studies of cancer, for ethical reasons most of what we know about photoimmunology comes from studies of chemical antigens and infectious organisms using mouse models. Two of these models are contact hypersensitivity (CHS) to synthetic antigens and delayed type hypersensitivity (DTH) responses to bacterial products. The skin is the largest organ of the body. It is also an immune organ. Besides the interdigitating network of antigen presenting Langerhans cells, and macrophages and lymphocytes that enter the dermis through lymph vessels, epidermal skin cells themselves can influence the immune response by their production of cytokines such as IL-10, TNF-alpha, and many others (Schwarz et al. 1994). Trans-urocanic acid, produced in keratinocytes from the metabolism of histidine, is a potent absorber of UV radiation, and protects DNA in keratinocyte nuclei from UV-induced damage. Isomerization of trans-urocanic to the cis isoform by UVB radiation renders the compound a potent immunosuppressor (DeFabo & Noonan, 1983). Cis-urocanic acid binds the serotonin receptor 5-HT2A, which is found on dendritic cells, T cells, B cells, and peripheral nerves, and may mediate UV-induced immune suppression by it action on these cells (Waltersheid et al. 2006).
One of the major targets of UV-induced immune suppression is the Langerhans APC in the skin. Normally, antigens encountered in the skin are taken up by APC and transported to the draining lymph nodes, where it is presented to effector T cells, inducing an immune response specific for that antigen. Upon exposure to even suberythemal (i.e., below the level required to give a mild sunburn) doses of UVB radiation, the majority of Langerhans cells either die or migrate from the UV-treated skin to the draining lymph node (Figure 2).
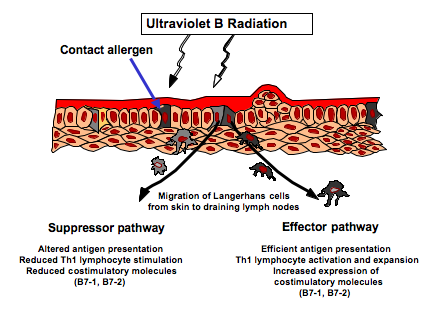
Figure 2. Effect of suberythemal doses of UV radiation on antigen presenting cells in the skin and their interaction with T cells in the draining lymph nodes.
The remaining Langerhans cells at the site of exposure exhibit damage (loss of dendricity and DNA damage), Figure 3. If a chemical antigen is painted on the site of UV exposure from 3 days to about 2 weeks later, it is taken up by these damaged APC and transported to the draining lymph nodes where the antigen-laden cells stimulate regulatory rather than effector T cells (Vink et al. 1996, Shreedhar et al. 1998, reviewed by Schwarz 2008).
Figure 3. UV radiation damages and depletes antigen presenting Langerhans cells in the skin. Photomicrograph of Langerhans cells (brown staining, indicated by arrows) in the shaved skin of mice before and after a suberythemal doses of UVB radiation (Strickland et al., 1994).
Erythmal doses (i.e., sufficient to cause a sunburn) of UV radiation cause increased blood flow to the irradiated skin. Cytokines and soluble factors from UV-damaged keratinocytes enter the circulation and exert systemic suppressive effects on the immune response (Figure 4).
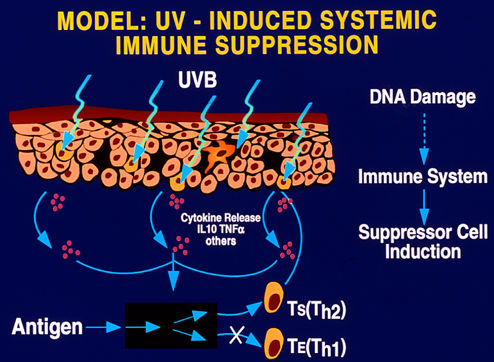
Figure 4. Erythemal doses of UV radiation induce systemic immune suppression by the release of cytokines, soluble mediators, and altered function of antigen presenting cells to induce antigen-specific regulatory T cells.
IL-10-secreting macrophages, attracted to the inflamed skin, suppress immune responses systemically (Hammerberg et al, 1996). Thus, unlike local suppression, which reduces the immune response to an antigen applied at the site of UV exposure but not to antigen applied to non-UV treated skin, IL-10 and other cytokines cause suppression of antigens encountered at both irradiated and non-UV irradiated sites. The systemic immune suppression is long lasting, antigen-specific and is mediated by class of lymphocytes called regulatory T cells (reviewed by Schwarz, 2008). Thus, UV-induced immune suppression is caused by both passive (damage to antigen presenting cells and subsequent inability to activate effector T cells) and active (induction of antigen-specific regulatory T cells) mechanisms. While the experiments described above were performed in laboratory animals, many of the same observations have been made in humans, and are reviewed by Norval et al. (2008). Lastly, the biologic effects of UV radiation depend on the waveband of UV and chromophore that absorbs the energy. UVB and UVA wavelengths have a complex interaction of biologic effects, which are reviewed by Halliday & Rana (2008).
Conclusion
The initial finding that wavelengths of light in the ultraviolet range of the solar electromagnetic spectrum could affect the immune response to UV-induced skin tumors led to the founding of a whole new branch of immunology: photoimmunology. In the 30+ years since it founding, the discoveries from many laboratories have contributed to the understanding of how UV radiation affects immune function, both at the local and systemic levels by clarifying the wavebands, chromophores, cellular components and cytokines involved in immune recognition and suppression. Together, the work has led to a better appreciation of the skin as an immune organ, and the role that environmental factors play in immunity.
References
DeFabo EC, Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J. Exp. Med. 158:84-98, 1983.
deGruijl FR. UV-induced immunosuppression in the balance. Photochem. Photobiol. 84:2-9, 2008.
Delves PJ, Martin S, Burton D, Roitt IM. Roitt's Essential Immunology. Pub. Wiley-Blackwell Publishers, NJ., USA. 2006.
Hammerberg C, Duriswamy N., Cooper KD. Temporal correlation between UV radiation locally-inducd tolerance and sequential appearance of dermal then epidermal class II MHC+CD11b+ monocyte/macrophage cells. J. Invest. Dermatol. 107:755-763, 1996.
Halliday GM, Rana S. Waveband and dose dependency of sunlight-induced immunomodulation and cellular change. Photochem. Photobiol. 84:36-46, 2008.
Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J. Natl. Cancer Inst. 53:1333-1336, 1974.
Norval M, McLoone P, Lesiak A, Narbutt J. The effect of chronic UV radiation on the human immune system. Photochem. Photobiol. 84:19-28, 2008.
Schwarz T, Urbanski A, Luger TA. Ultraviolet light and epidermal cell derived cytokines. In: Epidermal Growth Factors and Cytokines. (Ed. TA Luger and T Schwarz. Pp. 303-363. Marcel Dekker, New York.) 1994.
Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells - from disregarded T suppressor cells to highly respected regulatory T cells. Photochem. Photobiol. 84:10-18, 2008.
Shreedhar VK, Pride MW, Sun Y, Kripke ML, Strickland FM Origin and characteristics of UV-B radiation-induced suppressor T lymphocytes. J. Immunol. 161:1327-1335, 1998.
Strickland FM, Pelley RP, and Kripke ML. Prevention of ultraviolet radiation-induced suppression of contact hypersensitivity by Aloe barbadensis gel extract. J. Invest. Dermatol. 102:197-204, 1994.
Vink AA, Strickland FM, Bucana C, Cox PA, Roza L, Yarosh DB, Kripke ML. Localization of DNA damage and its role in altered antigen-presenting cell function in UV-irradiated mice J. Exp. Med. 183: 1491-1500, 1996.
Walterschid JP. Nghiem DX, Kazimi N, Nutt LK, McConkey DJ, Norval M, Ullrich SE. cis-Urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc. Natl. Acad. Sci. 103:17420-17425, 2006.
02/04/09