WHAT IS PHOTOBIOLOGY?
Kendric C. Smith
Emeritus Professor, Radiation Oncology (Radiation Biology)
Stanford University School of Medicine
800 Blossom Hill Road, Unit R169, Los Gatos, CA 95032
kendric@stanford.edu
web.stanford.edu/~kendric/
"Photobiology is broadly defined to include all biological phenomena involving non-ionizing radiation. It is recognized that photobiological responses are the result of chemical and/or physical changes induced in biological systems by non-ionizing radiation."
(Constitution of the American Society for Photobiology)
Non-ionizing radiation produces excited states in molecules due to the absorption of one or more photons. Excited-state molecules can react with adjacent molecules, but most frequently they undergo photochemical and photophysical changes within their own molecular structure. Non-ionizing radiation is grouped into three main regions; Ultraviolet (UV) radiation (short wavelengths that are not visible to man), Visible radiation (longer wavelengths than UV radiation), and Infrared radiation (still longer wavelengths, and also not visible to man).
The UV region is generally divided into three regions (especially in Photomedicine), i.e., the UV-C region, which is generally defined as being in the wavelength region from 100-280 nanometers (nm), the UV-B region as 280-320 nm, and the UV-A region as 320-400 nm. Other terms commonly used for the UV region are Far-UV (210-300 nm) and Near-UV (300-380 nm).
The Visible region is generally defined as 400-760 nm, and the Infrared region lies above 760 nm (Figure 1).
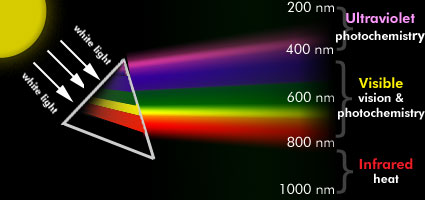
Figure 1. This figure depicts one of Newton's greatest discoveries: when white light from the sun passes through a prism, it is dispersed into a spectrum. Short wavelength radiation is highly refracted (deflected), and long wavelength radiation is only moderately refracted. Infrared radiation includes wavelengths longer than 800 nm, and its absorption causes only heating of the absorber. Visible radiation, with wavelengths from 400 nm to 800 nm, is important for vision and many other photobiological responses. Ultraviolet radiation, including wavelengths shorter than 400 nm, controls some photobiological reactions and many important photochemical reactions, but also has detrimental effects.
Light is composed of photons, and is propagated in the form of waves. Wavelength is the distance in the line of advance of a light wave from the bottom of one trough to the bottom of the next trough. [see Figure 1 in Basic Photochemistry] The photons at each wavelength have different energies; the shorter the wavelength (nm) the higher the energy.
The shortest wavelength of sunlight reaching the surface of the earth is at about 295 nm. Wavelengths shorter than this are filtered out by the stratospheric ozone layer. If the ozone layer is attenuated, then more short wavelength UV radiation will reach the earth, and will have a profound deleterious effect on man, animals, plants, and other organisms. The sun has both detrimental and beneficial effects on organisms, and all organisms have developed defense strategies (behavioral and biochemical) to protect themselves from the harmful wavelengths and harmful intensities, while optimizing the receipt of the beneficial wavelengths and intensities.
In general, the term "light" is used to define those wavelengths that are visible to man, although other organisms can "see" in the UV region. However, it is not uncommon for some people to use the term "UV light". UV radiation is the preferred term.
Figure 2 shows the spectrum of sunlight on earth during a typical day. Since life on earth evolved under the sun, whose terrestrial photon flux ("intensity") is greatest between 400 nm and 800 nm, it should not be surprising that most biological responses to light are induced by radiation between 400 nm and 800 nm. Very little radiation below 300 nm reaches the surface of the earth, because of its absorption by stratospheric ozone; very little radiation above 1000 nm reaches the surface of the earth because emission from the sun is low in this region, and because atmospheric water absorbs strongly above 1000 nm.
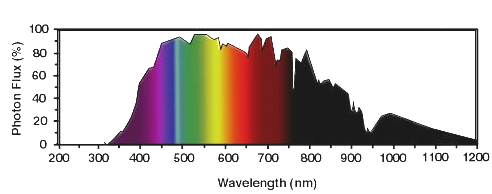
Figure 2. The spectrum of sunlight on earth on a clear summer day, from 300 to 1100 nm. The colors under the curve approximate those perceived by humans for each wavelength.
Photobiology is a large discipline that includes studies of both the beneficial and harmful effects of light. It covers topics from the atomic level to that of ecological communities. Photobiologists use all of the tools of science to study the chemical and biological effects of light and other non-ionizing radiation.
The importance of light is obvious when you look at the number of Nobel Prizes awarded for research in photobiology (Table 1).
1903 Niels R. Finsen
Physiology/Medicine: Phototherapy of lupus vulgaris and other diseases.
1911 Allvar Gullstrand
Physiology/Medicine: Dioptrics (image formation) of the eye.
1915 Richard M. Willstatter
Chemistry: The chemistry of chlorophyll and other plant pigments.
1930 Hans Fischer
Chemistry: The chemistry of heme pigments including chlorophyll.
1937 Paul Karrer
Chemistry: Studies on carotenoids, flavins and vitamins A and B2.
1938 Richard Kuhn
Chemistry: The chemistry of carotenoids and vitamins.
1961 Melvin Calvin
Physiology/Medicine: Carbon dioxide assimilation in plants (dark reactions).
1967 Ragnar Granit, Haldan K. Hartline, George Wald
Physiology/Medicine: Neurophysiology and the biochemistry of vision.
1981 David H. Hubel, Torsten N. Wiesel
Physiology/Medicine: Information processing in the visual system.
1988 Johann Deisenhofer, Robert Huber, Hartmut Michel
Chemistry: Structure of the photosynthetic reaction center.
1992 Rudolph A. Marcus
Chemistry: Theory of electron transfer reactions in chemical systems.
1995 Paul J. Crutzen, Mario J. Molino, F. Sherwood Rowland
Chemistry: Decomposition of stratospheric ozone by UV radiation.
1999 Ahmed Zewailv Chemistry: Femtosecond spectroscopy of transition states.
2002 John B. Fenn, Koichi Tanaka, Kurt Wuthrich
Chemistry: NMR and mass spectroscopy of biological molecules.
2008 Martin Chalfie, Osamu Shimomura, and Roger Y. Tsien
Chemistry: Discovery and development of the green fluorescent protein.
2012 Robert J. Lefkowitz and Brian K. Kobilka
Chemistry: For studies of G-protein-coupled receptors.
Table 1. Nobel Prizes in Photobiology
Photos and further information are available from the
Nobel Website. Search on a name.
The DIFFERENT SPECIALTY AREAS of PHOTOBIOLOGY
Photobiology can be divided into 13 major specialty areas. Twelve of these are concerned with the absorption of light in a biological system, and one is concerned with the emission of light by biological systems (Bioluminescence). These areas are briefly defined below, and will be more fully described in appropriate modules.
1. Photophysics. This specialty area is concerned with the physical interactions of light with matter at the atomic and molecular level. These include the vibration and rotation of molecules.
2. Photochemistry. This is the study of the chemical changes that occur in molecules after the direct absorption of light energy (compare with Photosensitization.). These include both alterations in the absorbing molecule, and reactions that occur between the absorbing molecule in its excited state and an adjacent molecule.
The First Law of Photochemistry states that "Light must be absorbed before photochemistry can occur". The power of this law is that by knowing the absorption spectrum of a molecule, i.e., by knowing which wavelengths of light can be absorbed by a molecule, one can immediately predict what wavelengths of light can have a photochemical effect on that molecule, and also what wavelengths of light will have no effect (since they are not absorbed).
3. Spectroscopy. The study of the absorption and emission of light by matter, as related to the dependence of these processes on the wavelength of the radiation. An Action Spectrum is the efficiency with which electromagnetic radiation produces a photochemical reaction, plotted as a function of the wavelength of the radiation. It shows which wavelengths of light are most effectively used in a specific chemical reaction (e.g., photosynthesis), and helps to identify the absorbing molecules.
4. Photosensitization. In this process, the light energy is absorbed by one type of molecule (the sensitizer), and the resulting energy-rich state(s) of the sensitizer then undergoes reactions that ultimately result in the chemical alteration of another type of molecule in the system (the substrate molecule). The sensitizer is not altered in certain types of photosensitization reactions.
Nearly all organisms contain molecules that are potential photosensitizers (e.g., bilirubin, chlorophylls, and porphyrins). Photodynamic therapy, which employs a photosensitizing drug and light, has important applications in cancer therapy. Certain forms of lung cancer and esophageal tumors are treated by photodynamic therapy to target and destroy cancer cells (see Photomedicine.).
5. UV Radiation Effects on Molecules and Cells. This field is concerned with the UV radiation photochemistry of deoxyribonucleic acid (DNA), ribonucleic acid (RNA) and proteins, and the biological effects produced by the photochemical and photophysical changes in these molecules (e.g., lethality, mutations). The field is also concerned with the sophisticated biochemical systems by which the cells can repair this photochemical damage.
6. Environmental Photobiology. The different wavelengths of sunlight exert both beneficial and detrimental effects, not only on individual cells and organisms, but more importantly on the whole ecosystem, where one deals with the effects of light on species composition and productivity.
7. Photomedicine. This field is concerned both with the detrimental effects and the beneficial effects of non-ionizing radiation. In Photomedicine, one most often thinks in terms of sunlight-induced skin cancer, but there are many other important topics. Then there is the beneficial area, where light alone, e.g. low level light therapy (LLLT), or sensitizers plus light are used to treat certain clinical conditions, e.g., psoriasis and cancer.
Photomedicine also includes the field of Photoimmunology, e.g., the absorption of light can modulate the immune system of the body, and thus prevent the immunological rejection of tumors.
8. Non-Visual Photoreception. Light is received by a receptor in an organism to monitor the environment without forming an image, in contrast to the case for Vision. A few examples are the circadian clock, which controls hormonal levels in birds and animals, and photoperiodism, which controls seasonal growth in plants and animals.
9. Vision. The photoreception that results in the formation of an image. This field covers the structure and photochemistry of the visual pigments in the rod and cone photoreceptors of eyes.
10. Photomorphogenesis. The development of an organism can be influenced by the information in light. This information comes from the quantity, the quality (i.e., wavelengths present), the spatial asymmetry (i.e., the direction from which the light comes), and the periodicity of the light. Some examples of photomorphogenesis are the germination of light sensitive seed, and the flowering of long-day plants.
11. Photomovement. To produce movement, plants and organisms depend upon the quality and the direction of the light striking their photoreceptors. In Photokinesis, an organism swims toward or away from light. Phototropic curvature in plants can occur toward or away from the light. Perhaps the best know example of this is sunflowers.
Charles Darwin, best known for developing the theory of evolution by natural selection, collaborated with his son Francis in writing an early book on phototropism, The Power of Movement in Plants (1880). This book has been so influential that Darwin would be well-known to biologists even if he had not written his great books on evolution.
12. Photosynthesis. It is not the information in the light that is used in photosynthesis, rather it is the energy of the light that is converted to stabilized chemical energy. This involves the absorption of light by a pigment, energy transfer, energy trapping or stabilization by reaction centers, and the initiation of chemical reactions from donor to acceptor molecules. This is a light harvesting reaction, while most of the other photobiological reactions require only a few photons to trigger responses.
13. Bioluminescence. For most people, bioluminescence is represented by the flash of a firefly or the phosphorescence that frequently occurs on agitating the surface of the ocean. Bioluminescence is the highly efficient cold-light emission that has a biological function for the organism concerned, e.g., finding a mate or food. More than half of all phyla in the animal kingdom contain members that are bioluminescent.
In Nature's Light, Francine Jacobs relates a story in which the bioluminescent fire beetle (Pyrophorus noctilucus) may have changed the history of the Americas. "In 1634, when the English were about to land at night on the island of Cuba, they saw many lights. Mistakenly, they believed them to be torches held by Spanish forces already on the island. Deciding that they were greatly outnumbered, the English withdrew and sailed on. What they probably observed were the glowing lights of fire beetles."
FINAL THOUGHTS
Sunlight is one of the most important elements in our environment. Plants harvest the energy of sunlight in order to grow, and thus to provide food for other organisms. Sunlight also provides information needed by organisms to trigger numerous biological responses. These are the beneficial wavelengths of sunlight.
Sunlight also has its bad side. The shorter wavelengths (or the longer wavelengths plus photosensitizers) can kill plants and other organisms, and produce cancer and other debilitating conditions in man.
Photobiology is the study of both the good and the bad effects of light. Studies range from the atomic level to the level of communities of organisms. Photobiologists use all of the tools of science to study the chemical and biological effects of light. Photobiology is an exciting and challenging field of science.
BOOKS on PHOTOBIOLOGY
Book Series by the European Society for Photobiology
JOURNALS
Photochemistry and Photobiology (ASP)
Photochemical & Photobiological Sciences (ESP)
10/12/08
11/09/09
06/02/11
09/08/13
03/18/14