THE PINEAL GLAND
A Neuroendocrine Transducer of Light Information
Anamika Sengupta and Gianluca Tosini
Circadian Rhythms and Sleep Disorders Program
Neuroscience Institute and Department of Pharmacology and Toxicology
Morehouse School of Medicine, Atlanta, GA 30310
anamika_sengupta@yahoo.com
gtosini@msm.edu
Introduction
The Pineal Gland (epiphysis cerebri in mammals), conferred as "the seat of Human soul" by Rene Descartes in 17th century, was considered as an epithalamic appendage of the vertebrate brain, and a vestigial evolutionary element until the 19th century. The endocrine aspect of the pineal gland reported in the early 20th century was confirmed through isolation of the hormone melatonin by Aaron B. Lerner in 1958. The pineal thus attracted scientific attention in the late 20th century due to its crucial role of transducing photoperiodic information through the rhythmic secretion of the daily and annual timekeeping hormone melatonin. It is now confirmed to be the neuroendocrine transducer of photic information into an endocrine response through the synthesis and release of the hormone melatonin.
Functional Organization of the Pineal Gland Exhibits a Unique Evolutionary Trend
The pineal gland develops from the roof of the embryonic forebrain and in adult brain. The pineal, together with habenula and the stria medullaris, constitutes the epithalamus. Evolutionary trends leading to the progressive replacement of direct photosensitivity (characteristic of non-mammalian vertebrates) by indirect photosensitivity (characteristic of mammals) have led to dramatic changes in the structure and function of the pineal gland (Falcon et al., 2009). The pineal gland of non-mammalian vertebrates is primarily composed of pinealocytes, which are structurally analogous to retinal cones, lower order neurons and interstitial glial cells (Collins et al., 1989; Falcon, 1999). The organization of the pineal in these animals resembles the vertebrate retina, albeit with much less complexity (Ekstrom and Miessl, 1997; Falcon 1999), and is indicative of its role as a luminance detector (Ekstrom and Miessl, 1997). In the mammalian pineal, the pinealocytes have lost the capability to directly respond to light, and their major function is the synthesis of melatonin (Falcon, 1999). However, it is worth noting that in neonatal rats and/or hamsters, the pinealocytes develop photoreceptor like characteristics that disappear after postnatal development birth (Clabough 1973; Zimmerman and Tso 1975). A series of studies have also shown that much of the photoreceptive machinery is still present in the pineal of neonatal mammals (Blackshaw and Snyder,1997), and that the pineal of neonatal rats may be capable of direct photosensitivity in vivo (Zweigg et al., 1966; Machado et al., 1969) and in vitro (Tosini et al., 2000; Fukuhara and Tosini 2003). Finally, it has also been suggested that melanopsin may mediate the photosensitivity observed in the neonatal pineal (Saafir et al., 2006). The reason why the mammalian pineal has lost the capability to directly respond to light is unknown, but it is believed to be a consequence of the evolutionary history of mammals (Menaker et al., 1997).
Melatonin Synthesis in the Pineal Gland
The pineal hormone melatonin is primarily synthesized in the pineal gland at night regardless of the diurnal or nocturnal activity of the animals. The initial step of the biosynthesis of this indoleamine involves the uptake of its precursor L-tryptophan from the circulation into the pinealocytes, followed by its conversion into 5-hydroxytryptophan by tryptophan hydroxylase. Further decarboxylation by L-aromatic aminoacid decarboxylase forms 5 hydroxytryptamine, also called serotonin. Acetylation of serotonin by serotonin N-acetyltransferase, also known as arylalkylamine N-acetyltransferase (AA-NAT), forms N-acetyl serotonin (NAS). Finally, methylation of N-acetylserotonin by hydroxyindole-O-methyltransferase (HIOMT) forms the final product melatonin (Figure 1). AA-NAT, the rate limiting enzyme for melatonin synthesis, activity is regulated at the transcriptional and post transcriptional levels (Klein et al., 1997; Gastel et al., 1998; Klein, 2007). In rats, pineal Aa-nat transcription and activity are up-regulated at night (100-fold more than during the day). By contrast, in monkeys and sheep, changes in the AA-NAT activity occurs at the protein level with small changes in the level of Aa-nat transcription (Coon et al., 1995; 2002).

Figure 1. Biosynthesis of melatonin from tryptophan in mammalian pinealocytes.
The suprachiasmatic nuclei (SCN) of the hypothalamus via a sympathetic multisynaptic pathway regulates rhythmic melatonin synthesis by acting on Aa-nat transcription (Klein et al., 1983; Tessonneaud et al., 1995; Garidou et al., 2002; Perreau-Lenz et al., 2005). The SCN uses a combination of daytime inhibitory and nighttime stimulatory signals to control the daily rhythm of pineal melatonin synthesis (Perreau-Lenz et al., 2003, 2004). At night the sympathetic neurotransmitter, Norepinephrine (NE), is released from the postganglionic nerve terminals innervating the pineal, thus stimulating (ß1 and
 1 adrenoreceptors on the pinealocytes, leading to an increase in intracellular calcium levels. This potentiates activation of adenylyl cyclase (AC) by a mechanism that involves protein kinase C (PKC) and calcium calmodulin protein kinase, followed by a 100-fold increase in intracellular levels of cAMP. High cAMP at night activates protein kinase A (PKA), which then phosphorylates AA-NAT, which forms a complex with 14-3-3 proteins (Fu et al., 2000), which protects it from dephosphorylation and denaturation, thus elevating melatonin synthesis in the pinealocytes (Ganguly et al., 2001; Schomerus and Korf 2005). Recent studies have also shown that the removal of the SCN results in a daytime increase in Aa-nat mRNA levels, suggesting that the presence of an inhibitory SCN output contributs to the control of pineal melatonin rhythm (Kalsbeek et al., 2000). It has also been suggested that the GABA-ergic output of the SCN terminals on to the pre-autonomic PVN neurons may be the daytime inhibitory signal contributing to the morning decline of melatonin synthesis (Kalsbeek et al., 2000).
1 adrenoreceptors on the pinealocytes, leading to an increase in intracellular calcium levels. This potentiates activation of adenylyl cyclase (AC) by a mechanism that involves protein kinase C (PKC) and calcium calmodulin protein kinase, followed by a 100-fold increase in intracellular levels of cAMP. High cAMP at night activates protein kinase A (PKA), which then phosphorylates AA-NAT, which forms a complex with 14-3-3 proteins (Fu et al., 2000), which protects it from dephosphorylation and denaturation, thus elevating melatonin synthesis in the pinealocytes (Ganguly et al., 2001; Schomerus and Korf 2005). Recent studies have also shown that the removal of the SCN results in a daytime increase in Aa-nat mRNA levels, suggesting that the presence of an inhibitory SCN output contributs to the control of pineal melatonin rhythm (Kalsbeek et al., 2000). It has also been suggested that the GABA-ergic output of the SCN terminals on to the pre-autonomic PVN neurons may be the daytime inhibitory signal contributing to the morning decline of melatonin synthesis (Kalsbeek et al., 2000).
Another important factor in the regulation of the melatonin level is represented by light. Light is the dominant environmental factor that regulates melatonin biosynthesis in vertebrates, and regardless of whether a species is diurnal/nocturnal or exhibits crepuscular activity, pineal melatonin levels are high during the dark phase of a natural or imposed illumination cycle (Reiter, 1991; Arendt, 1999). Exceptions to this rule are the retinal melatonin levels of Salmonoid fish (Iguchi et al., 1981; Arendt, 2005). Light signals perceived by the retina are conveyed via the retinohypothalamic tract to the SCN, and then to the mammalian pineal gland via the previously mentioned pathway (Korf et al., 1998; Korf, 1999). Light exerts a distinct suppressive effect on melatonin production irrespective of whether it is full spectrum white light, monochromatic light or UV-A light. The amount of light required to suppress melatonin production at night varies with species, previous light exposures, and the particular time of the night (Bojkowski et al., 1987; Brainard et al., 1988, 2001). Blue light (446-477 nm) is the most effective light to suppress melatonin (Brainard et al., 2001), thus suggesting that the intrinsically photosensitive retinal ganglion cells (ipRGCs) are involved in this phenomenon (Paul et al., 2009). Another important factor involved in the regulation of melatonin synthesis and levels is age. Melatonin or 6-sulphatoxtmelatonin levels in humans, barely detectable after birth, attains a robust rhythm between 6-8 weeks of age (Kennaway et al., 1992), followed by a rapid increase during puberty, and then a steady decrease during adulthood (Waldhauser et al., 1991; Karasek, 2004).
Metabolism of Melatonin
Once melatonin has been synthesized by the pinealocytes it freely diffuses out. Due to its amphiphilic nature, it can easily cross the blood brain barrier to enter the CNS and/or the general circulation (Hardeland, 2009; Leston et al., 2009). Several concurrent pathways are involved in the metabolism of melatonin. Melatonin in the CNS is metabolized by various isoforms of cytochrome P450 monooxygenase enzymes (CYPs) present in the brain (Ma et al., 2005; Pandi-Perumal et al., 2006). Isoforms of these enzymes (CYP1A2, CYP2C19) may either demethylate melatonin into NAS, or hydroxylation of melatonin into 6 hydroxymelatonin (through CYP1A1, CYP1B1) may occur, which may then be conjugated with sulphates to form 6-sulphatoxymelatonin. In the pineal gland melatonin, is deacetylated to 5-methoxytryptamine (5-MT; Rogowski et al., 1979; Beck and Jonsson 1981) with the help of enzymes called Aryl acrylamidases (AAAs), although 5-MT can be formed from serotonin also. The 5-MT is catabolised by monoamine oxidase in the pineal (Raynaud and Pevet, 1991). The third metabolic pathway is the kynurenine pathway (30% of overall melatonin metabolism) involving enzymes like myeloperoxidase, indoleamine 2, 3 dioxygenase and other non-enzymatic oxidants, which cleave the pyrrole ring of melatonin forming N1-acetyl-N2-formyl-5-methoxykinuramine (AFMK) and N1-acetyl-5-methoxy-kynuramine (AMK) (Hirata et al., 1974) These metabolic biproducts of melatonin help in scavenging reactive oxygen and nitrogen species, act as a mitochondrial modulator and regulator of cyclooxygnase-2 (Tan et al., 2001; Hardeland et al., 2009; Hardeland, 2010). The metabolized forms of melatonin, along with a small amount of unmetabolized melatonin, are excreted through the urine.
Melatonin Acts via G-Protein-Coupled Receptors
Melatonin is known to have autocrine and paracrine effects on various cell types and peripheral tissues. Such effects studied in vitro and in vivo have been found to be dependent on specific melatonin receptors (Jockers et al., 2008). In mammals, melatonin receptors so far identified include G protein-coupled melatonin receptor type 1 (MT1) and type 2 (MT2) (Reppert, 1997; Figure 2). Recent studies have also identified MT3 as quinone reductase 2, a detoxifying enzyme having functional homology with other quinone reductases in human tissues (Nosjean et al., 2000). The role played by MT3 in is still unclear.

Figure 2. Pathways activated by MT1 and MT2 receptor signaling. Both Melatonin receptors are negatively coupled with adenylyl cyclase (AC) and the activation of these receptors decrease cAMP. Mel = melatonin; K= potassium; BK= Big Potassium channels; PKA = Protein kinase A; cAMP = Cyclic adenosine monophosphate; P-CREB = phosphorilated-cAMP response element-binding element; PLC= Phospholipase C; DAG = diacylglycerol; PKC = Protein kinase C; GC = Guanylate cyclase; cGMP = Cyclic guanosine monophosphate.
The binding characteristics of MT1 and MT2 receptors are almost the same, MT2 having a kD value about 3 times lower than that of MT1. The distribution of MT1 and MT2 receptors in mammals is variable. MT1 and MT2 receptors are widely distributed in the body (Whitt-Enderby et al., 2003; Luchetti et al., 2010). MT1 is a 350 amino acid long protein exhibiting 60% structural homology with the 362 amino acid long MT2 receptor protein (Reppert, 1997). Both of the melatonin receptors have 7 transmembrane alpha helices with 4 intracellular and 4 extracellular domains, characteristic features of all G protein coupled receptors in mammals. Recent studies on the detailed structural properties of melatonin receptors (Rivara et al., 2005; Chugunov et al., 2006; Farce et al., 2008; Mazna et al., 2008 ) show that the dimensions of the putative ligand binding site, which lies on TM5 in both the receptors, is found to be relatively smaller in the case of MT1, when compared to MT2. This dissimilarity between the two melatonin receptors affects their binding affinity (Luchetti et al., 2010). The residues in the C terminal region of the MT receptors, specifically, TM7 exposed to the cytoplasmic face of the target cell, is the site for G protein binding (Jockers et al., 2008) and for the interaction with intracellular proteins. Proteins like filamin A (insulin receptor substrate 4) are common members of MT1 and MT2 associated complex (Daulat et al., 2007). Small GTPases like Rac1, Rap1A, 2',3'-cyclic nucleotide, 3' phosphodiesterase, protein elongation factor, 1-gamma, forms specific complexes with MT1 subtype, while catenin, delta-1, protein phosphatase (PP) 2C-gamma interacts specifically with MT2 (Daulat et al., 2007). The C terminal tails for both MT receptors contain a single cysteine residue (C7.72 and C7.77 for MT1 and MT2, respectively), essential for adenylate cyclase activity inhibition, while a Tyr residue, Y7.64, is found to influence receptor activity and internalization (Sethi et al., 2008). Phosphorylation sites of PKA, PKC and casein kinase 1, 2 are found in the C terminal cytoplasmic domain (Dubocovich and Markowska, 2005).
The AC/cAMP/PKA/CREB pathway is activated by melatonin binding to MT1 receptors (Jockers et al., 2008; Figure 2). A classical example of the involvement of this downstream signaling pathway is reflected in the acute endocrine effects of melatonin on the pars tuberalis of the pituitary (Fustin et al., 2009). Activation of MT1 receptor by melatonin increases the phosphorylation of mitogen activated protein kinase 2 (MEK2), extracellular signal-regulated kinases 1 and 2 (EKR1 and ERK 2) (Witt-Enderby et al., 2000), and c-jun-N-terminal kinase (JNK) via pertussis toxin sensitive and insensitive G protein (Chan et al., 2002).
The activation of MT2 receptors by the binding of its ligand inhibits forskolin stimulated cAMP production (Reppert et al., 1995; MacKenzie et al., 2002), thus stimulating phosphoinositide turnover (Chan et al., 2002; MacKenzie et al., 2002). Stimulation of phosphoinositide turnover by activation of the ßγ subunit of pertusis-toxin sensitive G protein by MT1 receptors has also been reported (Godson and Reppert, 1997; Roka et al., 1999). The GC/cGMP pathway is inhibited by MT2 receptor activation (Hernandez-Pacheko et al., 2008; Figure 2).
Melatonin signaling is hypothesized to be influenced by homo and heterodimerization of the MT1 and MT2 receptors (Levoye et al., 2006a; Jockers et al., 2008), and the coupling of these receptors to other G protein coupled receptors like GPR50 (G protein coupled orphan receptor 50), which have high homology with melatonin receptors, but designated as orphan receptors as they do not bind to melatonin, and their endogenous ligand is unknown (Jockers et al., 2008). They heterodimerize with MT1 and MT2, abolishing high affinity agonist binding and G protein coupling to MT1 (acting as MT1 agonist), but does not influence MT2 receptors (Levoye et al., 2006b).
Pineal Involvement in the Modulation of Circadian Rhythms
Melatonin acts as a chronobiotic molecule (Pevet et al., 2006), stabilizing or re-enforcing the circadian rhythms of body functions in mammals like rodents (Armstrong, 1989). Human studies on the phase shift of circadian rhythms of body functions, such as body temperature and sleep-wake cycle, are affected by melatonin. Phase advances follow the evening administration of melatonin, while phase delays follow morning administration (Lewy et al., 1992; Cajochen et al., 2003). Timed administration of melatonin has been shown to facilitate readjustments after acute phase shifts of the light-dark cycle, e.g., jet lag (Arendt et al., 1997). The chronobiotic effect of melatonin on circadian rhythms can be attributed to the influence of melatonin on the metabolic and electrical activity of the SCN, as shown in several in vitro and in vivo studies (Pevet et al., 2006; Kudo et al., 2007). Melatonin differentially influence the expression of an array of clock genes, notably Period (Per1, 2, 3) and Cryptochrome (Cry1, 2), which are expressed in the pars tuberalis and SCN (Lincoln et al., 2003; Agez et al., 2009). The activation of Per genes occurs during the early day, when melatonin levels are low, and the activation of Cry occurs during the early night, when melatonin levels are raising (Lincoln et al., 2003). The pattern of melatonin secretion conveys photoperiodic information to the pars tuberalis, which in turn influences the pattern of expression of the Per and Cry genes, translating the melatonin signal for entraining body rhythms (rhythmic synthesis and secretion of hormone) to the light phase of the external environment (Walton et al., 2011). These effects are melatonin and pineal dependent. A pinealectomy or the removal of MT1 receptors abolish such rhythms in the pars tuberalis (Messager et al., 2001; Von Gall et al., 2002).
Regulation of the Sleep-Wake Cycle
The sleep-wake cycle is regulated by the interaction of endogenous circadian and homeostatic processes in the body. Circadian rhythm sleep disorders arise when there is a misalignment between the timing of the endogenous circadian rhythms and the external environment, or when there is dysfunction of the circadian clock or its entrainment pathways (Dodson and Zee, 2010). Research over the last decade has established a strong relationship between melatonin levels and sleep homeostatic mechanisms in humans (Rajaratnam et al., 2004). The quality of sleep declines drastically with aging, as do the nocturnal levels of melatonin in humans (Pandi-Perumal et al., 2005). Moreover, patients with insomnia or experimental animals induced with insomnia (rapid wakefulness and inability to sleep between 2 a.m - 3 a.m) or narcolepsy or hyperactive disorder in children, can be corrected by the administration of pharmacological oral doses of melatonin ranging between 5-50 mg (Cardinali et al., 2002). Administration of melatonin at bedtime is found to resynchronize circadian rhythms with sleep function, thus promoting sleep (Cardinali et al., 2002; Arendt, 2003). Sleep disturbances associated with jetlag can be corrected by the oral administration of melatonin during bedtime between 10 pm and midnight (Brzezinski et al., 2005). Thus, from these reports we can conclude that the timed administration of exogenous melatonin can be useful in the treatment of certain circadian rhythm sleep disorders, including delayed sleep phase, advanced sleep phase, free-running, and irregular sleep-wake cycle.
However, the role of endogenous melatonin in the regulation of the sleep-wake cycle in mammals is still questionable. Surgical pinealectomy in rats housed under a 12:12 light-dark cycle did not affect REM or NREM sleep (Rechtschaffen et al., 1969), although pinealectomised rats housed in constant darkness exhibited a decrease in the amplitude of the circadian rhythm of REM and NREM sleep (Kawakami et al., 1972). Such studied indicate that circulating endogenous melatonin may have a modest effect on sleep or diurnal organization of sleep-wake cycles in mammals like rats (Meldenson and Bergmann, 2001; Fisher and Sugden, 2010). The exogenous administration of melatonin or its agonists is widely used as a treatment of insomnia, however, the question about mechanistic aspects of the involvement of exogenous melatonin in sleep promotion remains an open question. A recent study has reported that the administration of IIK7, an MT2 subtype selective agonist, has an acute sleep-promoting action in the rat, which is very similar to that seen after the administration of melatonin, suggesting that an MT2 melatonin receptor subtype mediates the acute hypnotic effect of melatonin (Fisher and Sugden, 2009)
Photoperiodic Responses
Physiological time measurement is linked to photoperiodism (Figure 3). The pineal gland via melatonin mediates photoperiodic time measurement in mammals (Malpaux et al., 2001; Hezlerigg 2010). Pinealectomy is reported to block photoperiodic responses in all experimental mammals studied so far (Hezlerigg and Wagner, 2006). In mammals, environmental light information is received by the classical retinal photoreceptors (rods and cones), or ipRGCs is conveyed via the SCN (Card et al., 1991; Lucas et al., 1999; Paul et al., 2009) to the pineal gland, which via melatonin regulates seasonal photoperiodic responses in many mammalian species (Bartness et al., 1993; Bittman and Karsch, 1984). Long duration melatonin signals promote winter physiology, while short duration signals promote summer physiology (Lincoln et al., 2005). The infusion of melatonin in pinealectomised mammals activated photoperiod dependent seasonal physiology (Bartness et al., 1993).
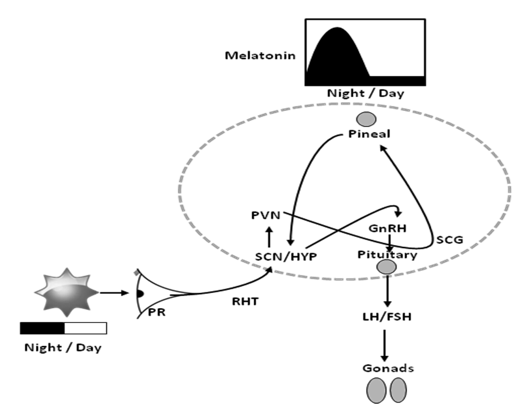
Figure 3. Light perceived through the retinal photoreceptors (PR), and transmitted through the retino-hypothalamic tract (RHT), affects mammalian reproduction via a complex pathway that involves the pineal, the suprachaismatic nucleus (SCN) of the hypothalamus (HYP), the paraventricular nuclei, (PVN), superior cervical ganglion (SCG), and the pituitary gland. Hormones secreted include gonadotropin releasing hormone (GnRH) from the hypothalamus, leutinizing hormone (LH), and follicle stimulating hormone (FSH) from the pituitary, which affects gonadal activity.
Exposure of Syrian hamsters to short day lengths (less than 12.5 hr of light) decreases the blood concentration of gonadotropins (FSH, LH) and sex steroids (progesterone, testosterone), which leads to gonadal regression (by 10%) followed by reproductive quiescence for about 16-20 weeks, which corresponds to the duration of autumn and winter (Prendergast et al., 2009). During this time of gonadal recrudescence the gonads become photorefractory (gonadal conditions are unlinked to photoperiodic inhibition), a condition that enables the gonads to become functional in spring before the environmental photoperiods return to 12.5 hr. Photorefractoriness can be terminated by the exposure of animals to long day lengths (Butler et al., 2010). To answer if melatonin is involved in this process, studies with microimplants of melatonin in the SCN and parts of the thalamus of photorefractory Siberian hamsters for 12 weeks during exposure to long daylengths were capable of blocking the dissipation of photorefractoriness by long daylengths (Teubner et al., 2008). However, a recent study by Butler et al., (2010) has shown that the seasonal transition to summer photosensitive phenotype, which is essential for reproduction in seasonal breeding mammals, is melatonin independent.
The nightly duration of melatonin secretion is reported to affect reproduction by altering the steroid negative feedback effects on the hypothalamus and pituitary. In humans, melatonin down regulates the expression of hypothalamic GnRH in a cyclic pattern over a 24 hr period, an effect mediated by its G-protein coupled MT1 and MT2 receptors. This influences the pulsatile secretion of gonadal steroids, namely follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary. A decline in melatonin concentration below the threshold level (115 pg/ml) is thus essential for the initiation of puberty (Juszczak and Michalska, 2006), while an early decline in melatonin concentration may activate the hypothalamo-gonadal axis, leading to precocious puberty in humans (Commentz and Helmke, 1995). The fact that young boys with pineal tumors show precocious puberty is indicative of the involvement of the pineal (Silman et al., 1979) and melatonin in this process (de Holanda et al., 2010). The molecular mechanism underlying the role of melatonin during puberty can be explained through recent investigations, which highlight the fact that members of the Rfamide family of peptides like kisspeptine and gonadotropin inhibiting hormone (GnIF), which have antogonastic effects on hypothalamic GnRH neurons (kisspeptin stimulating and GnIF inhibiting GnRH secretion), is influenced by melatonin (Smith and Clarke, 2007). Melatonin increases GnIF secretion and inhibits kisspeptin secretion in cultured mammalian hypothalamic neurons (Gingerich et al., 2009).
The direct MT1 and MT2 mediated effect of melatonin on human ovarian granulosa cells influences LH secretion and ovarian lutinization (Woo et al., 2001). Melatonin treatment increases human chorionic gonadotropin stimulated progesterone secretion by the corpus luteum through inhibition of ovarian GnRH (Stocco et al., 2007). Taken together, these studies indicate the involvement of pineal and melatonin in the regulation of reproduction in seasonally reproducing mammal, as well as in humans.
Melatonin and Glucose metabolism
New data have also suggested a role for melatonin in the regulation of carbohydrate metabolism (Peschke, 2008), and indeed, a recent study using melatonin receptor knock-out mice has indicated an active role of these receptors in the regulation of blood glucose (Muhlbauer et al., 2009). A recent paper has also reported that melatonin treatment can improve glucose metabolism in an insulin-resistant mouse model by restoring the action of insulin on the vasculature (Sartori et al., 2009). Additional support for a role of melatonin in the regulation of glucose metabolism has been provided by a series of studies that have linked melatonin receptor type 2 in the pathogenesis of type 2 diabetes (Bouatia-Naji et al., 2009; Lyssenko et al., 2009). Finally, a recent investigation has shown that the removal of MT1 induces insulin resistance in the mouse (Contreras-Alcantara et al., 2010). The mechanism by which melatonin affects glucose metabolism is still under investigation, but it is believed that melatonin may modulate insulin secretion by the pancreas, and glucose uptake by skeletal muscle.
Conclusion
Extensive studies over the last 50 years have shown that the pineal gland and melatonin play an important role in the modulation of many aspects of mammalian physiology. Although this article attempts to highlight the versatile role of pineal and melatonin in the regulation of mammalian physiology, in no way can it summarize the entire spectrum of physiological functions regulated by the pleiotropic hormone melatonin or pineal. Further studies are necessary for the full elucidation of the functional role of the mammalian pineal and its principle indole melatonin.
References
Agez L, Laurent V, Guerrero HY, Pvet P, Masson-Pvet M, Gauer F. 2009. Endogenous melatonin provides an effective circadian message to both the suprachiasmatic nuclei and the pars tuberalis of the rat. J Pineal Res. 46: 95-105.
Arendt J, Skene DJ, Middleton B, Lockley S.W Deacon S. 1997. Efficacy of melatonin treatment in jet lag, shift work, and blindness, J. Biol. Rhythms 12: 604-617.
Arendt J. 1999. Is melatonin a photoperiodic signal in humans? Adv. Exp. Med. Biol. 460: 417-424.
Arendt J. 2003. Importance and relevance of melatonin to human biological rhythms. J Neuroendocrinol. 15: 427-431.
Arendt J. 2005. Melatonin: characteristics, concerns, and prospects. J. Biol. Rhythms 20: 291-303.
Armstrong SM. 1989. Melatonin: the internal zeitgeber of mammals, Pineal Res. 7: 157-202.
Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. 1993. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J. Pineal Res. 15: 161-190.
Beck O, Jonsson G. 1981. In vivo formation of 5-methoxytryptamine from melatonin in rat. J. Neurochem. 36: 2013-2018.
Bittman EL, Karsch FJ. 1984. Nightly duration of pineal melatonin secretion determines the reproductive response to inhibitory day length in the ewe. Biol. Reprod. 30: 585-593.
Blackshaw S, Snyder SH. 1997. Developmental expression pattern of phototransduction components in mammalian pineal implies a light-sensing function. J. Neurosci. 17: 8074-8082.
Bojkowski CJ, Aldhous ME, English J, Franey C, Poulton AL, Skene DJ, Arendt J. 1987. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Horm. Metab. Res. 19: 437-440.
Bouatia-Naji N, Bonnefond A, Cavalcanti-Proena C, Sparsø T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chvre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jørgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Lévy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. 2009. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 41: 89-94.
Brainard GC, Lewy AJ, Menaker M, Fredrickson RH, Miller LS, Weleber RG, Cassone V, Hudson D. 1988. Dose response relationship between light irradiance and the suppression of plasma melatonin in human volunteers. Brain Res. 454: 212-218.
Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. 2001. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J. Neurosci. 21: 6405-6412.
Butler MP, Turner KW, Park JH, Schoomer EE, Zucker I, Gorman MR. 2010. Seasonal regulation of reproduction: altered role of melatonin under naturalistic conditions in hamsters. Proc. Biol. Sci. 277: 2867-2874.
Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, Ben-Shushan A, Ford I. 2005. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med. Rev. 9: 41-50.
Cajochen C, Krauchi K, Wirz-justice A. 2003. Role of melatonin in regulation of human circadian rhythms and sleep. J. Neuroendocrinol. 15: 432-437.
Card JP, Whealy ME, Robbins AK, Moore RY, Enquist LW. 1991. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron 6: 957-969.
Cardinali DP, Brusco L I, LLoret SP and Furio AM. 2002. Melatonin in sleep disorders and jet lag. Neuroendocrine Lett. 1: 9-13.
Chan AS, Lai FP, Lo RK, Voyno-Yasenetskaya TA, Stanbridge EJ, Wong YH. 2002. Melatonin MT1 and MT2 receptors stimulate c-Jun N-terminal kinase via pertussis toxin-sensitive and insensitive G proteins. Cell Signal. 14: 249-257.
Chugunov AO, Farce A, Chavatte P, and Efremov RG. 2006. Differences in binding sites of two melatonin receptors help to explain their selectivity to some melatonin analogs: a molecular modeling study. J. Biomol. Struct. Dyn. 24: 91-107.
Clabough JW. 1973. Cytological aspects of pineal development in rats and hamsters. Am. J. Anat.137: 215-229
Collin JP, Voisin P, Falcón J, Faure JP, Brisson P, Defaye JR. 1989. Pineal transducers in the course of evolution: molecular organization, rhythmic metabolic activity and role. Arch. Histol. Cytol. 52: 441-449.
Commentz JC, Helmke K. 1995. Precocious puberty and decreased melatonin secretion due to a hypothalamic hamartoma. Horm. Res. 44: 271-275.
Contreras-Alcantara S, Baba K, Tosini G. 2010. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity 18: 1861-1863.
Coon SL, Roseboom PH, Baler R, Weller JL, Namboodiri MA, Koonin EV, Klein DC. 1995. Pineal serotonin N-acetyltransferase: expression cloning and molecular analysis. Science 270: 1681-1683.
Coon SL, Del Olmo E, Young WS 3rd, Klein DC. 2002. Melatonin synthesis enzymes in Macaca mulatta: focus on arylalkylamine N-acetyltransferase. J. Clin. Endocrinol. Metab. 87: 4699-4706.
Daulat AM, Maurice P, Froment C, Guillaume JL, Broussard C, Monsarrat B, Delagrange P, Jockers R. 2007. Purification and identification of G protein-coupled receptor protein complexes under native conditions. Mol. Cell. Proteomics 6: 835-844.
de Holanda FS, Tufik S, Bignotto M, Maganhin CG, Vieira LH, Baracat EC, Soares JM. 2010. Evaluation of melatonin on the precocious puberty: a pilot study. Gynecol Endocrinol. (doi: 0.3109/09513590.2010.501888).
Dodson ER, Zee PC. 2010. Therapeutics for Circadian Rhythm Sleep Disorders. Sleep Med Clin. 5: 701-715.
Dubocovich ML, Markowska M. 2005. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 27: 101-110.
Ekstrom P, Meissl H. 1997. The pineal organ of teleost fishes. Rev. Fish. Biol. Fish. 7: 199-284.
Falcón J. 1999. Cellular circadian clocks in the pineal. Prog. Neurobiol. 58:121-162.
Falcón J, Besseau L, Fuents M, Sauzet S, Magnanou E, Boeuf G. 2009. Structural and functional evolution of the pineal melatonin system in vertebrates. Ann. N.Y. Acad. Sci. 1163: 101-111.
Farce A, Chugunov AO, Loge C, Sabaouni A, Yous S, Dilly S, Renault N, Vergoten G, Efremov R G. Lesieur D. Chavatte P. 2008. Homology modeling of MT1 and MT2 receptors. Eur. J. Med. Chem. 43: 1926-1944.
Fisher SP, Sugden D. 2009. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci. Lett. 457: 93-96.
Fisher SP, Sugden D. 2010. Endogenous Melatonin is Not Obligatory for the Regulation of the Rat Sleep- Wake Cycle. Sleep 33: 834-840.
Fu H, Subramanian RR, Masters SC. 2000. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 40:617-647.
Fukuhara C, Tosini G. 2003. Induction of photosensitivity in cultured rat pineal affects Aa-nat regulation. Brain Res Dev Brain Res. 142: 219-221.
Fustin JM, Dardente H, Wagner GC, Carter DA, Johnston JD, Lincoln GA, Hazlerigg DG. 2009. Egr1 involvement in evening gene regulation by melatonin. FASEB J. 23: 764-773.
Ganguly S, Gastel JA, Weller JL, Schwartz C, Jaffe H, Namboodiri MA, Coon SL, Hickman AB, Rollag M, Obsil T, Beauverger P, Ferry G, Boutin JA, Klein DC. 2001. Role of a pineal cAMP-operated arylalkylamine N-acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proc Natl Acad Sci USA. 98: 8083-8088.
Garidou ML, Gauer F, Vivien-Roels B, Sicard B, Pévet P, Simonneaux V. 2002. Pineal arylalkylamine N-acetyltransferase gene expression is highly stimulated at night in the diurnal rodent, Arvicanthis ansorgei. Eur. J. Neurosci. 15: 1632-1640.
Gastel JA, Roseboom PH, Rinaldi PA, Weller JL, Klein DC. 1998. Melatonin production: proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science 279: 1358-1360.
Gingerich S, Wang X, Lee PK, Dhillon SS, Chalmers JA, Koletar MM, Belsham DD. 2009. The generation of an array of clonal, immortalized cell models from the rat hypothalamus: analysis of melatonin effects on kisspeptin and gonadotropin-inhibitory hormone neurons. Neuroscience 162: 1134-1140.
Godson C, Reppert SM. 1997. The Mel (1a) melatonin receptor is coupled to parallel signal transduction pathways. Endocrinology 138: 397-404.
Hardeland R. 2009. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors 35: 183-192.
Hardeland R, Tan DX, Reiter RJ. 2009. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 47: 109-126.
Hardeland R. 2010. Melatonin metabolism in the central nervous system. Curr. Neuropharmacol. 8: 168-181.
Hazlerigg DG, Wagner GC. 2006. Seasonal photoperiodism in vertebrates: from coincidence to amplitude. Trends Endocrinol. Metab. 17: 83-91.
Hazlerigg D. 2010. Genetic and molecular mechanisms of mammalian photoperiodism, in: R.J. Nelson, D.L. Denlinger, D.E. Somers (Eds.), Photoperiodism: The Biological Calendar, Oxford University Press, Oxford; New York 543-560.
Hernandez-Pacheco A, Araiza-Saldana C I, Granados-Soto V, Mixcoatl-Zecuatl T. 2008. Possible participation of the nitric oxide-cyclic GMP-protein kinase G-K channels pathway in the peripheral antinociception of melatonin. Eur.J. Pharmacol. 596: 70-76.
Hirata F, Hayaishi O, Tokuyama T, Senoh S. 1974. In vitro and in vivo formation of two new metabolites of melatonin. J. Biol.Chem. 249: 1311-1313.
Iguchi H, Kato KI, Ibayashi H. 1981. Melatonin serum levels and metabolic clearance rate in patients with liver cirrhosis. J. Clin. Endocrinol. Metab. 54: 1025-1027.
Jockers R, Maurice P, Boutin JA, Delagrange P. 2008. Melatonin receptors, heterodimerization, signal transduction and binding sites: what's new? Br. J. Pharmacol. 154: 1182-1195.
Juszczak M, Michalska M. 2006. The effect of melatonin on prolactin, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) synthesis and secretion. Postepy Hig. Med. Dosw. 60:431-438.
Kawakami M, Yamaoka S, Yamaguchi T. 1972. Influence of light and hormones upon circadian rhythm of EEG slow wave and paradoxical sleep. In: Itoh S, Ogata K, Yoshimura H, eds. Advances in Climatic Physiology. Tokyo, Japan: Igaku Shoin; 349-366.
Kennaway DJ, Stamp GE, Goble FC. 1992. Development of melatonin production in infants and the impact of prematurity. J. Clin. Endocrinol. Metab. 75: 367-369.
Klein DC, Smoot R, Weller JL, Higa S, Markey SP, Creed GJ, Jacobowitz DM. 1983. Lesions of the paraventricular nucleus area of the hypothalamus disrupt the suprachiasmatic leads to spinal cord circuit in the melatonin rhythm generating system Brain Res. Bull. 10: 647-652.
Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Bégay V, Falcón J, Cahill GM, Cassone VM, Baler R. 1997. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog. Horm. Res. 52: 307-357.
Kalsbeek A, Garidou ML, Palm IF, Van Der Vliet J, Simonneaux V, Pvet P, Buijs RM. 2000. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur. J. Neurosci. 12: 3146-3154.
Karasek M. 2004. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 39: 1723-1729.
Klein DC. 2007. Arylalkylamine N-acetyltransferase: "the Timezyme". J. Biol. Chem. 282: 4233-4237.
Korf HW, Schomerus C, Stehle JH. 1998. The pineal organ, its hormone melatonin, and the photoneuroendocrine system. Adv. Anat. Embryol. Cell Biol. 146: 1-100.
Korf HW. 1999. Evolution of melatonin producing-pinealocytes. Adv. Exp. Med. Biol. 460: 17-29.
Kudo T, Horikawa K, Shibata S. 2007. Circadian rhythms in CNS and peripheral clock disorders.The circadian clock and hyperlipidemia. J. Pharmacol. Sci. 103: 139-143.
Lerner AB, Chase JD, Takahashi Y, Lee TH, Mori N. 1958. Isolation of melatonin, pineal factor that lightens melanocytes. J. Am. Cham. Soc. 80: 2587-2589.
Leston J, Harthé C, Brun J, Mottolese C, Mertens P, Sindou M, Claustrat B. 2009. Melatonin is release in the third ventricle in humans. A study in movement disorders. Neurosci. Lett. 469: 294-297.
Levoye A, Jockers R, Ayoub MA, Delagrange P, Savaskan E, Guillaume JL. 2006a. Are G protein-coupled receptor heterodimers of physiological relevance?-Focus on melatonin receptors. Chronobiol. Int. 23: 419-426.
Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, Jockers R. 2006b. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 25: 3012-3023.
Lewy AJ, Ahmed S. Latham Jackson JM, Sack RL. 1992. Melatonin shifts human circadian rhythms according to a phase response curve, Chronobiol. Int. 9: 380-392.
Lincoln GA, Andersson H, Hazlerigg D. 2003. Clock genes and the long-term regulation of prolactin secretion: evidence for a photoperiod/circannual timer in the pars tuberalis. J. Neuroendocrinol. 15: 390-397.
Lincoln GA, Johnston JD, Andersson H, Wagner G, Hazlerigg DG. 2005. Photorefractoriness in mammals: dissociating a seasonal timer from the circadian-based photoperiod response. Endocrinology 146: 3782-3790.
Luchetti F, Canonico B, Betti M, Arcangeletti M, Pilolli F, Piroddi M, Canesi L, Papa S, Galli F. 2010. Melatonin signaling and cell protection function. FASEB J. 24: 3603-3624
Lucas RJ, Freedman MS, Muoz M, Garcia-Fernndez JM, Foster RG. 1999. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 284: 505-507.
Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spgel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. 2009. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 41: 82-88.
Ma X, Idle JR, Krausz KW, Gonzalez FJ. 2005. Metabolism of melatonin by human cytochromes p450. Drug. Metab. Dispos. 33: 489-494.
Machado CR, Wragg LE. 1969. Circadian rhythm of serotonin in the pineal body of immunosympathectomized immature rats. Science 164: 442-443.
MacKenzie RS, Melan MA, Passey DK, Witt-Enderby PA. 2002. Dual coupling of MT1 and MT2 melatonin receptors to cyclic AMP and phosphoinositide signal transduction cascades and their regulation following melatonin exposure. Biochem. Pharmacol. 63: 587-595.
Malpaux B, Migaud M, Tricoire H, Chemineau P. 2001. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J. Biol. Rhythms 16: 336-347.
Mazna P, Grycova L, Balik A, Zemkova H, Friedlova E, Obsilova V, Obsil T, Teisinger J. 2008. The role of proline residues in the structure and function of human MT2 melatonin receptor. J. Pineal Res. 45: 361-372.
Menaker M. Moreira LF. Tosini G. 1997. Evolution of circadian organization in vertebrates. Braz. J. Med. Biol. Res. 30: 305-313.
Mendelson WB, Bergmann BM . 2001. Effects of pinealectomy on baseline sleep and response to sleep deprivation. Sleep 24: 369-373.
Messager S, Garabette ML, Hastings MH, Hazlerigg DG. 2001. Tissue-specific abolition of Per1 expression in the pars tuberalis by pinealectomy in the Syrian hamster. Neuroreport 12: 579-582.
Mühlbauer E, Gross E, Labucay K, Wolgast S, Peschke E. 2009. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur. J. Pharmacol. 606: 61-71.
Nosjean O, Ferro M, Coge F, Beauverger P, Henlin J M, Lefoulon F, Fauchere J L, Delagrange P, Canet E, Boutin JA. 2000. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 275: 31311-31317.
Pandi-Perumal SR, Zisapel N, Srinivasan V, Cardanali DP. 2005. Melatonin and sleep in ageing population. Exp Gerontol. 40: 911-925.
Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. 2006. Melatonin - Nature's most versatile biological signal? FEBS J. 273: 2813-2838.
Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP. 2008. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 85: 335-353.
Paul KN, Saafir TB, Tosini G. 2009. The role of retinal photoreceptors in the regulation of circadian rhythms. Rev Endocr Metab Disord. 10: 271-278.
Peschke E. 2008. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 44: 26-40.
Perreau-Lenz S, Kalsbeek A, Garidou ML, Wortel J, van der Vliet J, van Heijningen C, Simonneaux V, Pvet P, Buijs RM. 2003. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 17: 221-228.
Perreau-Lenz S, Pvet P, Buijs RM, Kalsbeek A. 2004. The biological clock: the bodyguard of temporal homeostasis. Chronobiol. Int. 21: 1-25.
Perreau-Lenz S, Kalsbeek A, Van Der Vliet J, Pvet P, Buijs RM. 2005. In vivo evidence for a controlled offset of melatonin synthesis at dawn by the suprachiasmatic nucleus in the rat. Neuroscience 130: 797-803.
Pévet P, Agez L, Bothorel B, Saboureau M, Gauer F, Laurent V, Masson-Pvet M. 2006. Melatonin in the multi-oscillatory mammalian circadian world. Chronobiol. Int. 23: 39-51.
Prendergast BJ, Zucker I, Nelson RJ. 2009. Seasonal rhythms of mammalian behavioral neuroendocrinology, in: D.W. Pfaff (Ed.), Hormones, Brain, and Behavior, Elsevier/Academic Press, Amsterdam/Boston. pp. 507-538.
Rajaratnam SM, Middleton B, Stone BM, Arendt J, Dijk DJ. 2004. Melatonin advances circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in human. J. Physiol. 560: 229-351.
Raynaud F, Pévet P. 1991. 5-Methoxytryptamine is metabolized by monoamine oxidase A in the pineal gland and plasma of golden hamsters. Neurosci. Lett. 123: 172-174.
Rechtschaffen A, Whitehead WE, Whitehead PK, Wincor MZ. 1969. Role of the pineal gland in light-off triggering of paradoxical sleep in the rat. Psychophysiology 6: 272.
Reiter RJ. 1991. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 12: 151-180.
Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. 1995. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel(1b) melatonin receptor. Proc. Natl. Acad. Sci. USA 92: 8734-8738.
Reppert SM. 1997. Melatonin receptors: molecular biology of a new family of G protein-coupled receptors. J. Biol. Rhythms 12: 528-531.
Rivara S, Lorenzi S, Mor M, Plazzi P V, Spadoni G, Bedini A, Tarzia G. 2005. Analysis of structure-activity relationships for MT2 selective antagonists by melatonin MT1 and MT2 receptor models. J. Med. Chem. 48: 4049-4060.
Rogawski MA, Roth RH, Aghajanian GK. 1979. Melatonin: deacetylation to 5-methoxytryptamine by liver but not brain arylacylamidase. J. Neurochem. 32: 1219-1226.
Roka F, Brydon L, Waldhoer M, Strosberg AD, Freissmuth M, Jockers R. 1999. Tight association of the human Mel(1a)-melatonin receptor and G(i): precoupling and constitutive activity. Mol. Pharmacol. 56: 1014-1024.
Saafir T.B., MacLeish P. R., Tosini G. 2006. Induction of photosensitivity in the mammalian pinealocytes. Society for Neuroscience Annual Meeting Abstract
Sartori C, Dessen P, Mathieu C, Monney A, Bloch J, Nicod P, Scherrer U, Duplain H. 2009. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology 150: 5311-5317.
Sethi S, Adams W, Pollock J, Witt-Enderby PA. 2008. C-terminal domains within human MT1 and MT2 melatonin receptors are involved in internalization processes. J. Pineal Res. 45: 212-218.
Schomerus C, Korf HW. 2005. Mechanisms regulating melatonin synthesis in the mammalian pineal organ. Ann. NY. Acad. Sci. 1057: 372-383.
Silman RE, Leone RM, Hooper RJ, Preece MA. 1979. Melatonin, the pineal gland and human puberty. Nature 282: 301-303.
Smith JT, Clarke IJ. 2007. Kisspeptin expression in the brain: catalyst for the initiation of puberty. Rev. Endocr. Metab. Disord. 8: 1-9.
Stocco C, Telleria C, Gibori G. 2007. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 28: 117-149.
Tan DX, Manchester LC, Burkhardt S, Sainz RM, Mayo JC, Kohen R, Shohami E, Huo YS, Hardeland R, Reiter RJ. 2001. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant FASEB J. 15: 2294-2296.
Tessonneaud A, Locatelli A, Caldani M. 1995. Bilateral lesions of the suprachiasmatic nuclei alter the nocturnal melatonin secretion in sheep. J. Neuroendocrinol. 7: 145-152
Teubner BJ, Smith CD, Freeman DA. 2008. Multiple melatonin target tissues mediate termination of photorefractoriness by long day lengths in Siberian hamsters. J. Biol. Rhythms 23: 502-510.
Tosini G, Doyle S, Geusz M, Menaker M. 2000. Induction of photosensitivity in neonatal rat pineal gland Proc Natl Acad Sci USA 97: 11540-11544.
Von Gall C, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm-Draeger PM, Weaver DR, Korf HW, Hastings MH, Stehle JH. 2002. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat. Neurosci. 5: 234-238.
Waldhauser F, Boepple PA, Schemper M, Mansfield MJ, Crowley WF Jr. 1991. Serum melatonin in central precocious puberty is lower than in age-matched prepubertal children. J. Clin. Endocrinol. Metab. 73: 793-796.
Witt-Enderby PA, MacKenzie RS, McKeon RM, Carroll EA, Bordt SL, Melan MA. 2000. Melatonin induction of filamentous structures in non-neuronal cells that is dependent on expression of the human mt1 melatonin receptor. Cell Motil. Cytoskeleton 46: 28-42.
Witt-Enderby P A, Bennett J, Jarzynka M J, Firestine S, Melan M A. 2003. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life. Sci.72: 2183-2198.
Woo MM, Tai CJ, Kang SK, Nathwani PS, Pang SF, Leung PC. 2001. Direct action of melatonin in human granulosa-luteal cells. J. Clin. Endocrinol. Metab. 86: 4789-4797.
Zimmerman BL, Tso MO. 1975. Morphologic evidence of photoreceptor differentiation of pinealocytes in the neonatal rat. J Cell Biol. 66: 60-75.
Zweig M, Snyder SH, Axelrod J. 1966. Evidence for a nonretinal pathway of light to the pineal gland of newborn rats. Proc. Natl. Acad. Sci. USA. 56: 515-520.
07/11/11