Photoreactivity of Drugs in vitro and in vivo
Hanne Hjorth Tønnesen1 and Steven W. Baertschi2
1School of Pharmacy, University of Oslo
P.O.Box 1068, Blindern, 0316 Oslo, Norway
h.h.tonnesen@farmasi.uio.no
2Baertschi Consulting, LLC
189 Twin Springs Court, Carmel, IN 46033
swbaertschi@aol.com
A drug substance or drug product can be exposed to natural or artificial light during production, storage, administration and use (Figure 1).
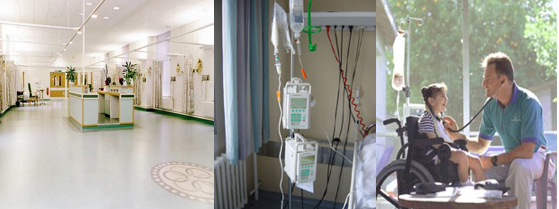
Figure 1. Environmental light conditions to which a drug substance or drug product can be exposed during production, storage and use. Left: fluorescent light tubes (emission mainly >400 nm). Middle: glass-filtered daylight (>310 nm). Right: direct sunlight (>290 nm).
As many drug substances and some formulation excipients (i.e., pharmacologically inactive ingredients in pharmaceutical preparations) absorb optical radiation in the UVB/UVA (290-400 nm) or visible (400-800 nm) part of the spectrum, they have the potential of being photoreactive in vitro and/or in vivo. The photoreactivity of a drug substance or drug product describes how efficiently and by which reaction pathway the compound or formulation responds to optical radiation. Absorption of optical radiation may result in the formation of free radicals and/or reactive oxygen species (i.e., singlet oxygen, superoxide, hydroxyl radical, peroxides, peroxy radicals, etc.). The photoinduced reactions might or might not be identical in vitro and in vivo, depending on the environment and chemistry of the absorbing species. Basic knowledge on the reaction mechanisms for the individual drug substances and products is important to ensure safe handling, packaging and labeling of the products, to reduce the potential for adverse effects, and to optimize drug therapy by, e.g., developing new drug delivery systems, formulations, or therapeutic regimes. The present module describes major aspects of interactions between drugs and radiation; i.e., drug photostability in vitro, photoinduced drug delivery in vivo, and photoinduced side effects in vivo.
Drug Photostability in vitro
A drug product consists of the active pharmaceutical ingredient (API), and one or more pharmaceutical excipients. Interactions between the API and optical radiation can result in the decomposition of the drug substance itself, or that of the excipients in the formulation through indirect (sensitized) reactions (Albini and Fasani, 1998). The most obvious result of the first process is a loss of potency of the product, which will lead to a loss of therapeutic effect of the preparation, and the formation of photodegradation products, which may or may not have associated toxicity concerns. Sensitized decomposition of other formulation components may lead to a change in physicochemical properties of the drug product (e.g., viscosity, droplet and particle size and charge), which may influence the physical stability of the preparation. This might have serious consequences for, e.g., emulsions or suspensions, particularly if the product is intended for parenteral (i.e., injection) administration (Tønnesen, 2004). The excipients, impurities, degradation products or dilution medium may also act as a photosensitizer to induce decomposition of the API. Such reactions can be very complex and difficult to elucidate. This is illustrated by the following example. Epinephrine (adrenaline) is given as a long term infusion for epidural analgesia after major surgery. The compound does not absorb optical radiation above 300 nm, and is therefore expected to be photostabile under indoor conditions. The substance turns out to be photostabile in all commonly used infusion media except for sterile glucose solution. A pure glucose solution does not absorb radiation above 300 nm. Glucose infusion medium is, however, sterilized by heat prior to the addition of adrenalin or other drug substances. During the heat sterilization a degradation product of glucose is formed i.e., 5-hydroxymethyl-2-furaldehyde (5-HMF). This degradation product has an absorption spectrum that extends above 300 nm, i.e., the compound is capable of absorbing radiation from glass-filtered daylight, and is efficient in producing singlet oxygen (Brustugun et al., 2005a) (Figure 2).
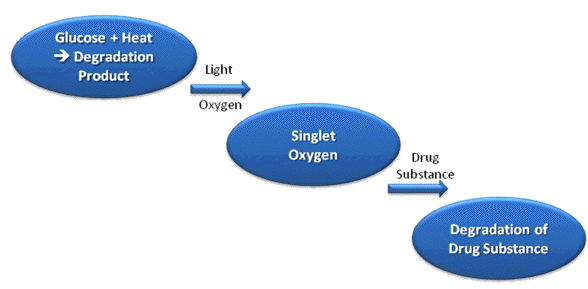
Figure 2. Photosensitized degradation of a drug substance in glucose infusion medium. A thermal degradation product of glucose (5-hydroxymethyl-2-furaldehyde) is formed during heat sterilization of the infusion medium. The degradation product is a very efficient photosensitizer for the formation of singlet oxygen, which can attack the drug substance and cause degradation.
Many drug substances, among them catecholamines, are susceptible to attack by singlet oxygen. This leads to the degradation of the drug substance. The concentration of 5-HMF allowed in sterile glucose infusion solutions exceeds by far the level needed to induce degradation of drugs present in the preparations. It is very difficult to avoid the formation of this product during the sterilization process. Every unit of glucose infusion medium thereby contains varying levels of a source that can photosensitize the formation of singlet oxygen. The photosensitized degradation of drugs diluted in glucose solutions is therefore a significant risk unless the units are protected against radiation in the UVB/UVA range (290-400 nm), which is present in glass-filtered sunlight, as well as many indoor lighting sources. Nearly 500,000 units of sterile glucose solution are used in Norwegian hospitals annually. This example also emphasizes the need for photostability testing of the final preparation, and not only the drug substance (Brustugun et al., 2005b).
Drug products can be exposed to different types of optical radiation during storage and use, e.g., in a hospital setting. In most cases the drug product would primarily experience exposure to visible light (400-800 nm; e.g., cool white fluorescent tubes) prior to distribution to the hospital pharmacy or ward, or to the home patient. It is, however, important to realize that all lamps, even incandescent and compact fluorescent bulbs, emit some radiation in the UVA-region (320-400 nm) of the spectrum. Situations of major concern are:
• Storage of drug preparations in hospital wards
In a hospital setting, drugs are often stored in unit-dose containers on an open shelf, in a refrigerator with constant lighting or even on the windowsill. In many cases, the protective market pack is removed; the inner container can be made of transparent plastic material or glass that offers little if any protection towards UV- and visible-radiation. The unprotected drug product can then be exposed to fluorescent tubes, and/or glass-filtered daylight for several days or even weeks prior to administration to the patient.
• Long term infusion regimes
Long-term infusion can lead to exposure for hours up to 2-3 days, and portable drug delivery devices allow the patient to bring the product outside the building. Degradation can also take place in the plastic tubing. A relatively large surface-to-volume of the drug solution is exposed, compared to the situation in the infusion bag. The actual precaution to be taken in the case of parenteral products is to protect the preparation against exposure, by using an outer bag (e.g., protection of the infusion bag), or by wrapping the infusion set (including the tubing) in aluminum foil. Both alternatives are time- and cost-consuming. The required action will in each case depend on the photochemical half-life of the drug substance (as a function of the particular light source) in the formulation.
• In situ preparation of parenteral products
The photoreactivity of a drug substance is dependent on its concentration in the actual preparation, and on the type of infusion medium or pharmaceutical formulation. A dilution step is often involved when preparations are made at the bedside, and adjusted for the individual patient (ex tempore, i.e, improvised preparation). Dilution (e.g., change of medium and change in concentration) may lead to altered requirements with respect to protection of the infusion set. A change in dilution medium from, e.g., sodium chloride to glucose can have a large impact on photostability, as described above.
Reactions in solution and in the solid state are often different for a variety of reasons, including differences in molecular mobility, imposed by the crystal lattice, or because the reaction occurs only at the crystal surface. Because photodegradation is a surface phenomenon, solid dosage forms are typically more stable than solutions. Solid state photochemical reactions are often related to a change of appearance of, e.g., a tablet, and might be observed by discoloration. A change in color is, typically associated with very low, difficult to detect, levels of chemical degradation of the drug substance. Thus, the photochemically-induced discoloration of tablets may result in unacceptable appearance changes even though the tablets meet all chemical specifications related to the purity of the drug substance. One example is yellow riboflavin tablets, which appear green after light exposure, although they still are quantitatively sound. It has been observed that the photostability of a drug substance can be dependent on its crystal modification (Sue-Chu et al., 2009).
Prevention of photodegradation or photostabilization of a drug product can be a relevant topic for consideration. Although there are various approaches to increase the photostability, as outlined in Figure 3, the task can be complex and is not always straightforward..
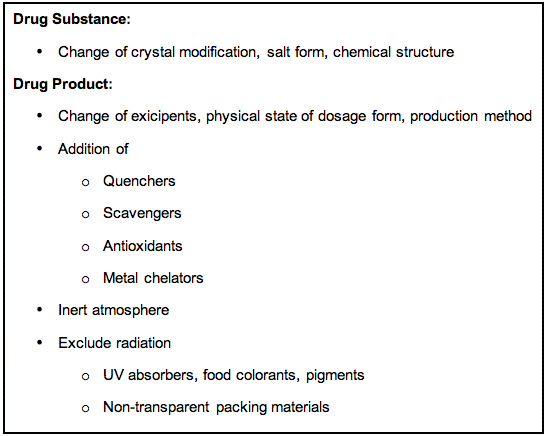
A change in the chemical structure of the API, or a change in the crystal modification or salt form of the drug substance might improve the photostability, but may also influence the bioavailability of the drug in an unwanted direction. Other options include a change in the physical state of the preparation (e.g., solution to tablet), or the addition of relevant excipients like quenchers, scavengers, antioxidants and/or metal chelators. A quencher is a molecular entity that deactivates (quenches) an excited state of another molecular entity, either by energy transfer, electron transfer, or by a chemical mechanism. A scavenger is a compound that reacts with the photochemically-induced reactive species, e.g., free radicals or reactive oxygen species. The addition of quenchers and scavengers to pharmaceutical preparations can be limited by the safety or pharmaceutical acceptability of many of these substances. Sulphites are frequently used as antioxidants in pharmaceutical preparations to prevent thermal oxidation of the API in the absence of light. Stabilization of catecholamines in parenteral preparations by the addition of metabisulphite is one example (Brustugun et al., 2004). In this case, the presence of sulphite did, however, have a photodestabilizing effect on these preparations, as illustrated in Figure 4. Trace amounts of adrenochrome, which is formed by the thermal degradation of epinephrine (adrenaline), forms an adduct with sulphite (adrenochrome sulphonate) that absorbs UVA-radiation (320-400 nm). The complex is an efficient photosensitizer of singlet oxygen, which causes photodecomposition of epinephrine.
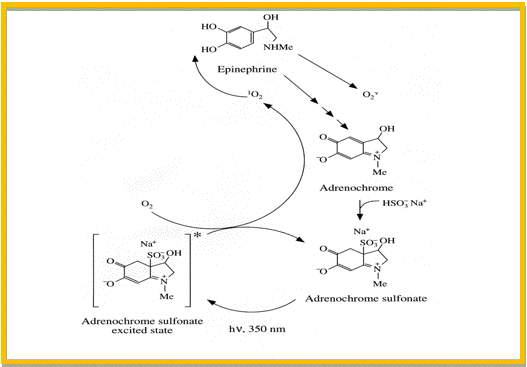
Figure 4. Photosensitized degradation of epinephrine in the presence of sulphite. First Step: adrenochrome (a thermal degradation product of epinephrine) reacts fast with sulphite to form adrenochrome sulphonate. Second Step: Adrenochrome sulphonate absorbs UVA-radiation and forms singlet oxygen. Final Step: Singlet oxygen attacks epinephrine, and causes degradation of the drug molecule.
Sulphites are therefore unsuitable as antioxidants in the case of catecholamines unless the preparation is protected from optical radiation through the production and product shelf-life. Metal chelators like EDTA are also reported to be pro-oxidants in certain photochemical reactions. The effect of the stabilizer can not be readily predicted, and therefore the final preparation should be tested. Because oxygen often participates in the photodegradation reactions, an inert atmosphere can often, but not always, improve the photostability. Unfortunately, this is not always the case. Nitrazepam forms strongly colored photodegradation products in the absence of oxygen, while it is photostabile under ambient oxygen pressure. A reliable way to secure a photostabile product is to minimize or exclude exposure to optical radiation. This can be obtained by the addition of UV absorbers, colorants and pigments to, e.g., a tablet coating or capsule shell, or to the packing material. The optical properties of two commonly used parenteral containers (presented in Figure 5) demonstrate that transparent packing materials offer little protection against optical radiation in the UV and visible range.
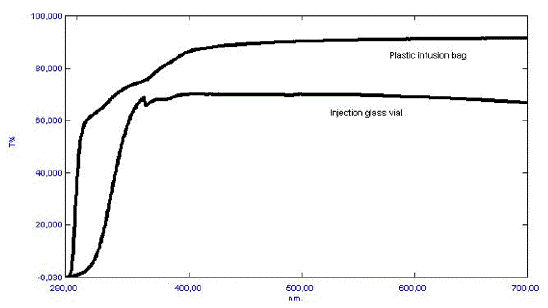
Figure 5. Optical properties (i.e., % Transmission) of a colorless glass injection vial, and a colorless plastic infusion bag.
The storage and use conditions seem to vary slightly between the USA, Europe and Japan, concerning the probability of drug exposure to glass-filtered daylight. In the USA and Japan, exposure to glass-filtered daylight is believed to occur infrequently in hospitals or pharmacies, while in Europe such exposure would be quite common, however, the situation is changing. In the USA, new cancer hospitals are designed to provide convenience, comfort and tranquility, with an architecture utilizing elements of natural light that "brings the outside inside" (e.g., a glass wall in the patient room). Energy saving efforts in the USA and EU are further contributing to the use of natural lighting. In the USA, as well as in Europe, there is also a trend towards the home treatment of elderly patients, and patients diagnosed with a chronic illness. Home infusion therapy service is now an important part of the clinically based care program offered in, e.g., the USA. Therefore, in the future, drug products will more likely to be exposed to glass-filtered daylight (>310 nm) or even direct sunlight (>290 nm) during storage and use.
Evaluation of in vitro photostability is essential to ensure adequate quality over the entire life span of the drug (Baertschi, 2005). Since January 1, 1998, it has been mandatory to perform photostability testing of new drug substances and drug formulations, according to the ICH (International Conference on Harmonisation) Guideline Q1B (1997) on photostability testing. The official regulations, however, do not cover drug products that were on the market before 1998, preparations under "in use" conditions (e.g., during infusion), or preparations that have been made for the individual patient (ex tempore preparations). In addition to calling for a revision of the current ICH photostability guideline, the need for guidance on in-use conditions was highlighted in a commentary by Baertschi et al. (2010). While there has been no efforts by ICH to take the Q1B photostability guidance through the revision process, a manuscript has been published that provides some guidance for the in-use considerations for pharmaceutical products for injection (Baertschi et al., 2013).
Photoinduced Drug Delivery in vivo
In some cases a benefit can be gained in vivo from the photoreactivity of a drug substance or a pharmaceutical formulation. The dominant examples in regular use are PUVA-therapy (combination of psoralens and UVA) as a treatment for psoriasis, application of blue light irradiation to counteract hyperbilirubinemia or neonatal jaundice, and photodynamic therapy (PDT) or photochemical internalization (PCI) as a treatment for various cancers. The latter topics are covered in other modules at this Website. Photodynamic therapy has, over the years, also received increasing attention as a viable option for the treatment of esophageal carcinoma and Barrett's esophagus, as well as age-related macular degeneration (AMD, the most common cause of blindness in the Western population), and in the treatment of atherosclerotic plaques. Another promising application of PDT is in the treatment of microbial infections. About 30% (i.e., 15 million per year) of the deaths worldwide are caused by infections. Drug resistance is reported in tuberculosis, dysentery, typhoid fever, gonorrhoea and a number of hospital infections caused by Staphylococcus aureus, Enterococcus spp. and Klebsiella pseudomonas.
PDT against microbial pathogens includes a broad spectrum of action, because singlet oxygen has a number of targets within the cell. For the same reason it is regarded less likely that the bacteria will develop resistance towards PDT (Jori and Coppellotti, 2007; Konopka and Goslinski, 2008). It is known that gram-positive bacteria species are much more sensitive to photodynamic inactivation (PDI) than gram-negative species. Efforts have therefore been made to design photosensitizers capable of attacking gram-negative strains. Antimicrobial PDT is making advances towards clinical applications within the field of oral infections, periodontal diseases, healing of infected wounds and treatment of Acne vulgaris. The first product to be applied in the oral cavity came on the market in Canada in 2005 (Periowave™, Ondine) and products for the treatment of infected wounds are under clinical trial. Antimicrobial PDT requires the topical application of photosensitizers, which are selective for the microorganism without causing unacceptable damage to the host tissue. The possibility of adverse effects on host tissues has often been raised as a limitation of antimicrobial PDT. However, studies have shown that the photosensitizers are more toxic against microbial species than against mammalian cells, and that the concentration of photosensitizer and light energy dose necessary to kill the infecting organism has little effect on adjacent host tissues. Photoactivated disinfection of blood samples and surfaces like benches and floors is also introduced as a promising application of antimicrobial PDT.
Finding optimal sensitizers and drug delivery systems is crucial in improving the efficacy, and reducing the side effects of PDT. Most of the candidate photosensitizers are porphyrin-like molecules (e.g., porphyrin derivatives, chlorines, bacteriocholins, phthalocyanins) possessing physicochemical properties that make them difficult to handle in the compounding process. They are polycyclic, often heavily charged and in many cases present as zwitterions, which makes them almost insoluble in both hydrophilic and lipophilic media. They are, thereby, difficult to incorporate in a simple emulsion or liposome preparation. In addition, they frequently form inactive aggregates in the presence of aqueous media. Alternative formulation approaches may therefore be required for these photosensitizers. Drug targeting from parenteral administration of photosensitizers has been achieved by using liposomes, oil emulsions, microspheres, micellar nanoparticles, proteins and monoclonal antibodies, but the ideal formulation has not yet been developed. Effort is being made to optimize the vehicle for topically administered sensitizers (e.g., dermal or to the oral cavity). A well-designed vehicle could allow the topical administration of sensitizers to tumors located close to the skin surface, thus offering an alternative to systemic administration. Application to the oral cavity and larynx would benefit from bioadhesive formulations to increase the contact time between sensitizer and tissue. The selection of vehicle also influences the localisation of sensitizer within the cells. This emphasizes the importance of optimizing the drug delivery system in order to increase bioavailability, target specificity, and reduce the side effects of products to be applied in PDT.
Novel drug delivery systems are directed toward a controlled release rate, a sustained duration of therapeutic action, and/or a targeting of the delivery to a specific tissue. One may take advantage of the photoreactivity of a pharmaceutical compound or excipient in the development of new drug delivery principles and devices. Photoactivation is an attractive option for triggering drug delivery, providing a broad range of adjustable parameters (e.g., wavelength, intensity, duration, spatial and temporal control) that can be optimized to suit a given application. Modern laser systems provide an energy confinement that makes them suitable for a number of biomedical applications. A combination of a laser and an optical fibre would allow very specific targeting to a tissue or organ. Laser excitation methods can therefore be used in a drug activating processes. Photoactivated drug delivery includes different approaches. The method can be used to control the release rate of the active principle from a dosage form (i.e., a carrier system), to activate a drug molecule that is already present at the site of action in an inactive form (e.g., a photosensitizer or a prodrug), or to combine the two (i.e., photocontrolled drug release and drug activation) (Sortino, 2008; Tønnesen, 2004). While photoactivation of sensitizers is well established, for instance in the treatment of cancer (photodynamic therapy, PDT) or psoriasis (PUVA-therapy), the application of photoactivated carrier systems offers an underdeveloped opportunity, and includes systems like photoresponsive hydrogels, microcapsules, liposomes, nanoparticles and oligonucleotides.
Photoinduced Side Effects in vivo
An increasing number of drug substances are reported to be photoreactive in vivo (Cosa, 2004; Ferguson, 2006; Schauder, 2005). Some drugs are photochemically inert in the sense that they do not decompose during exposure to optical radiation, yet still act as a source of free radicals, singlet oxygen, or the formation of phototoxic metabolites in vivo (Kleinman, 2010; Lynch, 2010). One example is nitrazepam (described above), which is photostabile under ambient oxygen pressure; however, under this condition the compound will generate singlet oxygen in combination with optical radiation, a process that that can result in a phototoxic reaction in vivo (Figure 6).
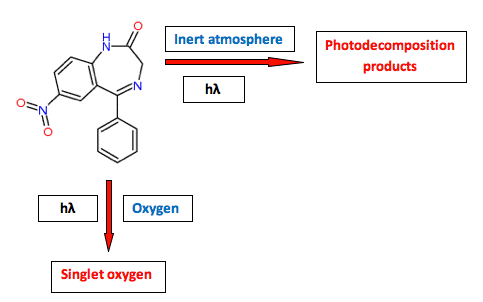
Figure 6. The photoreactivity of nitrazepam (a benzodiazepine drug) illustrates that a compound can be photostabile, but act as a photosensitizer, or it can be photolabile without a photosensitizing potential.
Adverse photosensitivity effects in patients manifest in responses that have been labeled as phototoxicity and photoallergy (Moore, 2002). Phototoxicity is defined as an alteration of cell function by an interaction between a chemical and non-ionizing radiation, the response being likened to an exaggerated sunburn. The reaction occurs upon simultaneous exposure to a phototoxic chemical (e.g., drug compound), and radiation of the appropriate wavelength. The wavelength of radiation implicated in most phototoxic reactions ranges from 300 to 400 nm. Most drug-induced phototoxic reactions are acute, occurring within a few minutes to several hours after exposure. They reach a peak from several hours to several days later, and usually disappear within a short time period after stopping either the drug or the exposure to radiation.
Photoallergy is an acquired altered capacity of the skin to react to light. Thus, it is immune-mediated wherein a drug molecule is stimulated by radiation to combine with a protein or other biomacromolecule in the skin to form an antigen. Photoallergy is believed to require exposure with an induction period before the response is observed, and it has a different histology compared to phototoxicity. A widespread exposure to artificial light sources, such as daylight lamps and solaria, a change in human leisure habits (i.e., more time spent outdoors), and the widespread use of drugs within the (Western) population increases the photosensitivity problem. Several requirements are, however, to be met if a drug is to cause a photosensitivity reaction. First, the drug substance or drug metabolite has to be distributed to tissues that are exposed to radiation, like the skin, eye and hair. Secondly, the UV-visible absorption spectrum of the drug substance or metabolite or their complexes with, e.g., proteins, must overlap the UV-visible transmission spectrum of the actual tissue. A fraction of the optical radiation above 300 nm will penetrate sufficiently deep into skin to react with substances circulating in the bloodstream, or accumulated in the tissue. The undesired photosensitivity reactions will occur in some people who either ingest the photosensitizing agents or apply them topically.
Enhanced ocular injury from UV radiation or visible light can be experienced in the presence of drugs that accumulate in the eye. However, cutaneous phototoxicity is by far the most common of the drug-induced photosensitivity reactions. Major skin reactions are erythema, hyperpigmentation, exaggerated sunburn and blisters. Drug-induced photosensitivity can be anticipated among a wide range of therapeutic groups, such as antidepressives, antihistamines, antihypertensives, antimicrobials, antineoplastics, antipsychotics, diuretics, hypoglycemic agents and nonsteroidal anti-inflammatory drugs (NSAIDs). Photosensitizing chemicals usually have a planar, tricyclic or polycyclic configuration, and a molecular weight <500 Daltons. The photochemical and photobiological mechanisms involve free radicals and/or singlet oxygen (Type I /Type II photochemical reactions). The same drugs are generally innocuous to skin in the absence of exposure to optical radiation. The main protective actions to be taken by the patient are to avoid exposure to direct sun and solaria, to use broad-spectrum sunscreens, to wear appropriate clothing and sunglasses, and to change medication if possible. Recent documents issued by the FDA and EMEA provide guidance on how to address photosafety assessments (i.e., in vitro testing) and labeling requirements for potentially in vivo photoreactive substances (FDA, 2003; Ibbotson, 2006; ICH S10, 2013). These guidelines are most likely to be applied to new drug substances, and not to products already on the market, but so far they are not mandatory.
Conclusion
Evaluation of interactions between drugs and optical radiation now forms a natural part of the research and development work for new drug substances and products; such information may be incomplete for drugs registered before 1998, and for ex tempore preparations. Basic knowledge on the photostability and photochemical reaction mechanisms for individual drug substances and products is required in order to ensure a safe handling, packaging and labeling of the products, to ensure safe and efficient medication, and to reduce costs. Knowledge about reaction mechanisms would further allow for the optimization of drug therapy by, e.g., development of new drug delivery systems or therapeutic regimes, and the reduction in adverse effects. The combination of drugs and optical radiation has a great potential in cancer therapy, and is likely to find new applications in the near future (e.g., in the battle against resistant bacteria).
References
Albini A, Fasani E (eds.) (1998). Drugs. Photochemistry and Photostability. The Royal Society of Chemistry, Cambridge.
Baertschi SW (Ed.) (2005) Pharmaceutical Stress Testing: Predicting Drug Degradation. Drugs and the Pharmaceutical Sciences, volume 153, Informa Healthcare, New York.
Baertschi SW, Alsante KM, Tønnesen HH (2010). A critical assessment of the ICH guideline on photostability testing of new drug substances and products (Q1B): Recommendation for revision. J Pharm Sci, 99: 2934-2940.
Baertschi SW, Clapham D, Foti C, Jansen PJ, Kristensen S, Reed RA, Templeton AC, Tønnesen HH (2013). Implications if in-use photostability: proposed guidance for photostability testing and labeling to support the administration of photosensitive pharmaceutical products, part 1: drug products administered by injection. J Pharm Sci, 102: 3888-3899.
Brustugun J, Kristensen S, Tønnesen HH (2004). Photostability of epinephrine - the influence of bisulfite and degradation products. Pharmazie, 59: 457-463.
Brustugun J, Tønnesen HH, Edge R, Navaratnam S (2005a). Formation and reactivity of free radicals in 5-hydroxymethyl-2-furaldehyde - the effect on isoprenaline photostability. J Photochem Photobiol B: Biol , 79: 109-119.
Brustugun J, Kristensen S, Tønnesen HH (2005b)Photosensitizing effect of 5-hydroxymethyl-2-furaldehyde in glucose infusion solutions. Pharmeuropa , 17: 460-461.
Cosa G (2004) Photodegradation and photosensitization in pharmaceutical products: Assessing drug phototoxicity. Pure Appl Chem 76: 263-275.
FDA, CDER (2003) Guidance for industry: photosafety testing. PDF
Ferguson J (2006) Drug-induced photosensitivity. In Ferguson J, Dover, JS, eds. Photodermatology, London: Manson Publishing, pp. 66-71.
Ibbotson S (2006) Photo-allergy and photopatch testing. In Ferguson J, Dover, JS, eds. Photodermatology, London: Manson Publishing, pp.72-80.
International Conference on Harmonisation (ICH) Q1B. Photostability testing of new drug substances and products (1997).
International Conference on Harmonisation (ICH) S10. Photosafety evaluation of pharmaceuticals (step 4) (2013).
Jori G, Coppellotti O (2007) Inactivation of pathogenic microorganisms by photodynamic techniques: mechanistic aspects and perspective applications. Anti-infective Agents Med Chem, 6: 119-131.
Kleinman MH, Smith MD, Kurali E, Kleinpeter S, Jiang K, Zhang Y, Kennedy-Gabb SA, Lynch AM, Geddes CD (2010) An evaluation of chemical photoreactivity and the relationship to phototoxicity. Reg Toxicol Pharmacol, 58: 224-232.
Konopka K, Goslinski T (2008) Prospects for photodynamic therapy in dentistry. Biophotonics Int, 15 (7): 32-35.
Lynch AM, Smith MD, Lane AS, Robinson SA, Kleinman MH, Kennedy-Gabb S, Wilcox P, Rees RW (2010) An evaluation of chemical photoreactivity and relationship to photogenotoxicity. Reg Toxicol Pharmacol, 58: 219-223.
Moore DE (2002) Drug-induced cutaneous photosensitivity. Incidence, mechanism, prevention and management. Drug safety 25: 345-372.
Schauder S (2005) Phototoxische Medikamente. Dtsch Apoth Zeit 145: 4287-4293.
Sortino S (2008) Nanostructured molecular films and nanoparticles with photoactivable functionalities. Photochem Photobiol Sci, 7: 911-924.
Sue-Chu M, Kristesen S, Tønnesen HH (2009) Photoinduced color changes in two different qualities of riboflavin in the solid state and in various tablet formulations. Photoreactivity of biologically active compounds. XX. Pharmazie, 64: 428-435.
Piechocki JT, Thoma K (2007) Pharmaceutical Photostability and Stabilization Technology. Drugs and the Pharmaceutical Sciences, volume 163. Informa Healthcare, New York.
Tønnesen HH (ed) (2004) Photostability of Drugs and Drug Formulations 2nd Ed. CRC Press, Boca Raton.
12/17/10
05/14/14