Molecular Evolution and Functional Diversity of Opsin-Based Photopigments
Takashi Nagata, Mitsumasa Koyanagi and Akihisa Terakita
Department of Biology and Geosciences, Graduate School of Science, Osaka City University, 3-3-138 Sugimoto-cho, Sumiyoshi-ku,
Osaka 558-8585, Japan
nagata@sci.osaka-cu.ac.jp
koyanagi@sci.osaka-cu.ac.jp
terakita@sci.osaka-cu.ac.jp
1. Introduction
Most animals capture light information by opsin-based photosensitive pigments (opsin pigments) for vision and non-visual functions. The opsin pigment consists of a protein moiety, opsin and a retinal chromophore (Figure 1).

Figure 1. The structure of bovine rhodopsin and chromophore retinal. (a) Rhodopsin consists of a protein moiety opsin and a chromophore retinal. The opsin has seven transmembrane alpha helices. Lys296 in helix VII covalently binds 11-cis-retinal via a Schiff base linkage. Protein Data Bank ID: 1GZM. (b) The chemical structures of the 11-cis and all-trans forms of retinal.
The opsin pigment is a typical G protein coupled receptor (GPCR) that binds to and activates various types of G proteins (guanine nucleotide-binding proteins) (Figure 2), and evolved from a non-photosensitive GPCR (Shichida and Matsuyama, 2009).
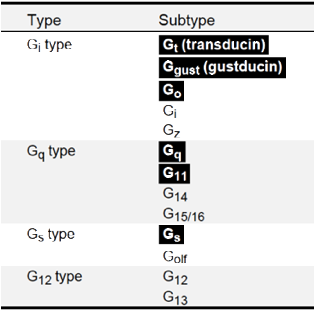
Figure 2. Diversity of G proteinsubunits involved in phototransduction cascades. Major animal G protein
subunits are roughly classified into four types (for further information, including the difinition of each G protein
subunit, see the review by Downes and Gautam, 1999). Mediators of phototransduction cascades, Gt(transducin), Ggust(gustducin), Go, Gq, G11 and Gs are highlighted. No opsin pigment has been found that couples to G12-type G proteins.
More than 2,000 opsin genes have been identified so far, showing diversity in the opsin family (Terakita, 2005; Musio and Santillo, 2009). In contrast to the diversity in the primary structure of opsins, it is widely accepted that they have similar seven transmembrane alpha helices, as first revealed by the crystal structure of the bovine visual pigment, rhodopsin (Palczewski et al., 2000) (Figure 1). The opsin-based pigments are often called rhodopsins, because vertebrate rhodopsins are the best understood opsin-based pigments. The opsin family is divided into eight subfamilies that are roughly discriminated by their molecular characteristics and/or functions (Figure 3).
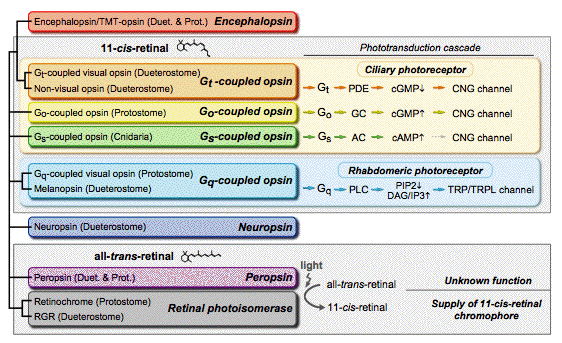
Figure 3. Phylogenic relationship and functional diversity of the eight subfamilies in the opsin family. Seven of the eight subfamilies (encephalopsin, Gt-coupled opsin, Go-coupled opsin, Gq-coupled opsin, neuropsin, peropsin, and retinal photoisomerase subfamilies) are composed of bilaterian opsins, and the other contains cnidarian opsins (Gs-coupled opsin subfamily). Gt-, Go-, Gs- and Gq-coupled opsin-based pigments, which bind to 11-cis-retinal as a chromophore, drive different phototransduction cascades mediated by Gt, Go, Gs or Gq, respectively. On the other hand, members of peropsin and retinal photoisomerase subfamilies bind to all-trans-retinal chromophore in the original dark state, and light isomerizes it to the 11-cis form. Retinochrome functions as a retinal photoisomerase, which regenerates 11-cis-retinal from all-trans-retinal (see also Figure 6). Note that the vertebrate non-visual opsin group contains opsin pigments that couple to various phototransduction cascades. The phototransduction cascade of Drosophila visual cells is shown as the Gq-mediated phototransduction cascade.
Below, we introduce molecular properties and functional characteristics of the members of each subfamily, and then discuss the molecular evolution and diversity of opsin-based pigments and photoreceptive systems.
2. Molecular Properties and Functional Characteristics of the Opsin Pigments
2.1. Vertebrate visual and non-visual opsin subfamily. In most vertebrates, two kinds of photoreceptor cells, rod and cone cells, mediate vision. They have different shapes and contain the following visual pigments composed of visual opsin and chromophores, i.e., rhodopsin and cone pigments, which are responsible for twilight vision in rod cells and daylight (color) vision in cone cells, respectively (Shichida and Imai, 1998). The cone opsins are divided into four subgroups, LWS, Rh2 (MWS), SWS1 and SWS2 pigment, which basically form red (longer wavelength) sensitive, green (middle wavelength) sensitive, blue (shorter wavelength) sensitive and UV/violet (shorter wavelength) sensitive pigments, respectively (Yokoyama, 1997; Terakita, 2005). Color vision is achieved with these cone opsin-based pigments with different color sensitivities.
Upon light absorption, rhodopsin and cone pigments convert to G-protein-activating photoproduct, called Meta II state, which has an absorption maximum at about 380 nm (Figure 4).
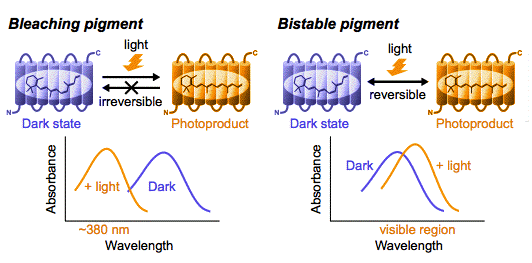
Figure 4. Comparison of photoreaction between bleaching pigment and bistable pigment. Photoproduct of bleaching pigments including vertebrate visual pigments, is thermally unstable and cannot revert to the dark state by light absorption, whereas photoproduct of bistable pigments, i.e., parapinopsin, Gq-coupled opsin pigment and Go-coupled opsin pigment, is thermally stable, and can be reconverted to the dark state by light absorption.
Meta II state is thermally unstable and eventually releases an all-trans-retinal chromophore (i.e., it "bleaches") (Matthews et al., 1963; Wald, 1968; Farrens and Khorana, 1995). Meta II states drive an enzyme cascade involving one of the Gi-type G proteins, transducin (Gt). Meta II states bind to and activate Gt, and the excited Gt in turn stimulates cyclic GMP (cGMP) phosphodiesterase, which hydrolyzes cGMP to GMP, resulting in a decrease in intracellular cGMP concentration (Figure 3). The decrease of cGMP concentration causes the closure of a cGMP-gated cation channel, a type of cyclic nucleotide-gated (CNG) channel, leading to hyperpolarization of the visual cell. Vertebrate rhodopsin and cone pigments are often called Gt-coupled rhodopsins, based on the G protein subtype with which they couple. In general, rods and cones contain similar but not identical sets of phototransduction molecules, i.e., transducin, cGMP phosphodiesterases and cGMP-gated channels as well as arrestin and kinase, both of which underlie termination of phototransduction (Hisatomi and Tokunaga, 2002), in addition to rhodopsin and cone pigments. The difference could be responsible for distinct kinetics of rod and cone photoresponses.
The visual pigments are also found in non-visual photoreceptor cells, including pineal photoreceptor cells of various non-mammalian vertebrates. On the other hand, non-visual photoreceptor cells in lower vertebrates also express non-visual opsin genes, i.e., pinopsin, parapinopsin, parietopsin and VA opsin (vertebrate ancient opsin).
Pinopsin and parapinopsin have been found in the pineal organ. Pinopsin is expressed in pineal photoreceptors and underlies photoreception by the pineal organs of birds (Okano et al., 1994; Max et al., 1995) and lizards (Taniguchi et al., 2001). Pinopsin activates both transducin and the G-protein G11 (see Figure 2), and therefore drives two different phototransduction cascades in the chicken pineal photoreceptor cells (Nakamura et al., 1999; Kasahara et al., 2002).
The parapinopsin gene and its expression were first identified in the catfish pineal and parapineal organs (Blackshaw and Snyder, 1997). We have shown that parapinopsin in the pineal organ of the lamprey (Koyanagi et al., 2004; Kawano-Yamashita et al., 2007) is a UV-sensitive pigment having two photointerconvertible stable states, the dark state and a thermally stable photoproduct with an absorption maximum in the visible region, which is called a bistable nature (Figure 4). The bistable nature is found in opsin pigments that belong to other subfamilies, i.e., Gq-coupled opsin pigments (composed of invertebrate visual pigments and melanopsin), and Go-coupled opsin pigment. Parapinopsin also exhibits a significantly lower G protein activation efficiency than that of bovine rhodopsin, like Go-coupled opsin pigment (Terakita et al., 2004). These facts suggest that parapinopsin has a sequence similarity with vertebrate visual opsins, but shares some molecular properties with opsin pigments of other subfamilies rather than vertebrate visual pigments. The intermediate characteristics of parapinopsin allow us to discuss an evolutionary scenario of vertebrate visual pigments as described in Section 3.
Parietopsin forms a green-sensitive pigment, and is expressed in photoreceptor cells of a lizard parietal eye (Su et al., 2006), which may be involved in the global detection of dawn and dusk (Solessio and Engbretson, 1993). The parietal-eye photoreceptor cells are unique, and have chromatically dependent hyperpolarizing and depolarizing responses to light (Solessio and Engbretson, 1993). It has been recently demonstrated that the hyperpolarizing and depolarizing responses are underlain by two kinds of signaling cascades, parietopsin-Go type G protein and pinopsin-gustducin cascades, respectively (Su et al., 2006).
VA opsin is found in amacrine and horizontal cells (two kinds of neural cells in the retina) of the salmon retina (Soni and Foster, 1997), but not in its rod and cone visual cells (Soni et al., 1998). A splice variant of VA opsin, called VAL opsin, is localized to deep parts of the brain and the horizontal cells of the zebrafish (Kojima et al., 2000). Recently, VA opsin is found in deep brain photoreceptors of chicken, and may mediate its photoperiodic response (Halford et al., 2009).
2.2. Gq-coupled visual opsins and melanopsin subfamily. Many higher invertebrates (protostomes) such as cephalopods and arthropods have visual opsins different from the vertebrate visual opsins (Figure 3). The visual opsin-based pigments light-dependently drive a phototransduction cascade mediated by Gq-type G protein but not transducin (Terakita et al., 1993, 1998; Lee et al., 1994; Yarfitz and Hurley, 1994). Therefore, the opsins and their pigments are called Gq-coupled visual opsins and Gq-coupled visual pigments (rhodopsins), respectively. The Gq-coupled visual pigments are localized in the microvilli of the rhabdomeric photoreceptor cells, which are morphologically distinct from the ciliary photoreceptor cells such as vertebrate rod and cone cells (Figure 5).
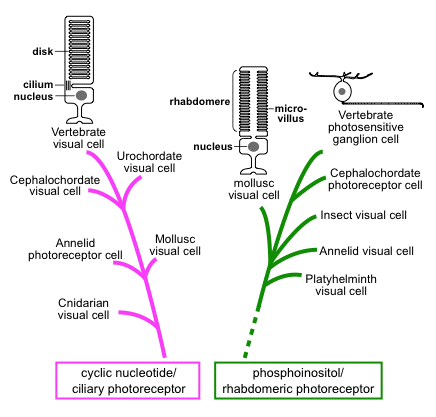
Figure 5. Phylogenetic understanding of animal photoreceptor cells. The animal photoreceptor cells are suggested to have evolved in the following two lineages: the ciliary photoreceptor cell employing cyclic nucleotide signaling (left), and the rhabdomeric photoreceptor cell employing phosphoinositol signaling (right).
As mentioned above, these pigments, in general, convert to thermally stable photoproducts with absorption maxima in the visible region by light absorption, and exhibit a bistable nature (Koyanagi and Terakita, 2008; Tsukamoto and Terakita, in press) (Figure 4). The photoproducts activate the Gq-type G protein, and it in turn stimulates phospholipase Cß, resulting in the depolarization of the photoreceptor cells (Yarfitz and Hurley, 1994; Yau and Hardie, 2009).
Pigments of different subgroups of insect and spider opsins have distinct absorption spectra. A set of visual opsins, each of which belongs to different subgroups, underlies insect and spider color vision. Phylogenetic analyses and sequence comparison of arthropod opsins, including insect and spider opsins, suggest that the ancestral arthropod could have at least trichromatic vision, with one UV-sensitive pigment and two visible light-sensitive pigments (Koyanagi et al., 2008a).
A major disadvantage of research on Gq-coupled visual pigments, compared with vertebrate visual pigments, is the difficulty in obtaining a large amount of purified protein using cultured cell systems. Instead, some insect opsins were analyzed in transgenic Drosophila whose photoreceptor cells heterologously express insect opsins (Zuker et al., 1988; Feiler et al., 1992; Townson et al., 1998; Engels et al., 2000). Recently, honeybee UV- and blue-sensitive visual pigments were successfully expressed in HEK293 cells, and purified pigments were spectroscopically investigated (Terakita et al., 2008). This made it possible to attempt mutational analyses of the Gq-coupled visual pigments, and compare structural and functional relationships between Gq-coupled visual pigments and other opsin pigments.
Melanopsin was first identified from the photosensitive dermal melanophores of Xenopus laevis (Provencio et al., 1998), and is found in a wide variety of vertebrates including human and other mammals. Melanopsin and invertebrate Gq-coupled visual opsins have a phylogenetically close relationship, and these two groups are in the Gq-coupled opsin subfamily (Koyanagi and Terakita, 2008). A series of knockout mice experiments revealed that melanopsin functions as a photopigment in intrinsic photosensitive retinal ganglion cells (ipRGCs), and is involved in non-image forming vision; i.e., pupil responses to light and photoentrainment of the circadian rhythm in mice (Hattar et al., 2003; Lucas et al., 2003; Panda et al., 2003).
In addition to non-image-forming visual functions, other possible functions of melanopsin have been recently discussed. In primates and mice, melanopsin regulates visual processing within the retina, probably optimizing visual pathways according to the time of day (Dacey et al., 2005; Barnard et al., 2006). It was also reported that melanopsin-expressing retinal ganglion-cells are diverse and can support spatial visual perception in mice (Ecker et al., 2010). Interestingly, most non-mammalian vertebrates possess more than two kinds of melanopsins, which are classified into two groups in the phylogenetic tree, but mammals have only one melanopsin gene (Bellingham et al., 2006). The physiological roles of two kinds of melanopsins in non-mammalian vertebrates remain uncertain.
We found melanopsin in amphioxus, and sea urchins also possess a melanopsin gene, demonstrating that deuterostomes have melanopsin gene(s), whereas protostomes have Gq-coupled visual pigment gene(s) (Koyanagi and Terakita, 2008). We successfully expressed amphioxus melanopsin in cultured cells, and investigated its molecular properties spectroscopically and biochemically. Amphioxus melanopsin forms a blue-sensitive photopigment with an absorption maximum around 485 nm (Koyanagi et al., 2005). The pigment shows a bistable nature and a light-dependent Gq activation in vitro with an efficiency similar to that of invertebrate Gq-coupled visual pigments (Terakita et al., 2008). In addition, amphioxus melanopsin is colocalized with a large amount of Gq in photoreceptor cells (Koyanagi et al., 2005). The Gq-containing photoreceptor cells show a depolarizing response to light, like invertebrate rhabdomeric photoreceptor cells (Gomez et al., 2009). These facts strongly support a putative signal transduction cascade; amphioxus melanopsin drives a Gq-mediated signal transduction cascade, like invertebrate Gq-coupled visual pigments. It is also suggested that mammalian melanopsin exhibits bistability in vivo, and drives a Gq/phosphoinositide-mediated phototransduction cascade (Fu et al., 2005; Isoldi et al., 2005; Panda et al., 2005; Qiu et al., 2005; Contin et al., 2006; Berson, 2007; Mure et al., 2007, 2009; Graham et al., 2008; although see Melyan et al., 2005).
2.3. Go-coupled opsin subfamily. Eakin has proposed that photoreceptor cells in animals can be classified into two morphologically distinct photoreceptor cell types, ciliary-type cells with membranes of modified cilia, and rhabdomeric-type cells with apical microvilli (Figure 5) (Eakin, 1965). Many higher invertebrates such as cephalopods and insects employ rhabdomeric photoreceptors, where Gq-coupled visual pigments function as photopigments, whereas some invertebrates possess ciliary photoreceptors. A new opsin, which is suggested to drive Go-type G protein, was first found in the scallop ciliary photoreceptors, and named Go-coupled opsin (Kojima et al., 1997). Scallop Go-coupled opsin is colocalized in the ciliary photoreceptors with a large amount of Go, and is suggested to activate Go in vivo. Consistently, the Go activation efficiency of bovine rhodopsin is significantly enhanced by replacement of the third cytoplasmic loop, which may be the basis of G protein subtype specificity (Yamashita et al., 2000), with the corresponding region of scallop Go-coupled rhodopsin (Terakita et al., 2002). Electrophysiological studies on the scallop ciliary photoreceptors suggest that scallop Go-coupled opsin pigment elevates the intracellular cGMP concentration through light-dependent activation of Go, which leads to hyperpolarization of the cell (Gomez and Nasi, 2000).
Go-coupled opsin was also found in amphioxus. Amphioxus Go-coupled opsin pigment exhibits an absorption maximum in the blue region when reconstituted with 11-cis-retinal (Koyanagi et al., 2002). Upon light absorption, amphioxus Go-coupled opsin pigment converts to a red-shifted photoproduct, which is thermally stable and reverts to the dark state with subsequent light absorption, and is therefore classified as a bistable pigment. Interestingly, amphioxus Go-coupled opsin binds to all-trans-retinal and forms a G-protein-activating state that appears to be spectroscopically and biochemically identical to the photoproduct (Tsukamoto et al., 2005).
2.4. Gs-coupled opsin subfamily. In ciliary photoreceptors in a cnidarian box jellyfish, the pigment of Gs-coupled opsin triggers a phototransduction cascade mediated by Gs (Koyanagi et al., 2008b). Jellyfish Gs-coupled opsin pigment is a green-sensitive pigment with 11-cis-retinal as a chromophore in the dark and photoconverts to a blue-shifted photoproduct with an absorption maximum in the visible region. The photoproduct is thermostable but does not revert to the dark state by subsequent light absorption, suggesting jellyfish Gs-coupled opsin pigment does not fit a criterion of bistable pigment. In the ciliary photoreceptor cells, jellyfish Gs-coupled opsin pigment is colocalized with a large amount of Gs and adenylyl cyclase. According to many studies on G protein-mediated signal transduction in higher animals, Gs activates adenylyl cyclase, which in turn causes CNG channel activation by increasing intracellular cAMP concentration (Simon et al., 1991; Firestein, 2001). Biochemical analyses revealed that the cAMP levels are elevated in a light-dependent manner in the jellyfish photoreceptors and cultured mammalian cells expressing jellyfish Gs-coupled opsin, indicating that Gs-coupled opsin pigment triggers a Gs-mediated signal transduction cascade that includes Gs and adenylyl cyclase. Gs-coupled opsins have been found also in other cnidarians including sea anemone and hydra (Plachetzki et al., 2007; Alvarez, 2008; Kozmik et al., 2008; Suga et al., 2008), and Gs-coupled opsin of hydra, which has extraocular photosensitivity (Taddei-Ferretti and Musio, 2000), is co-expressed with a CNG channel, suggesting the hydra also employs the Gs-mediated phototransduction cascade (Plachetzki et al., 2010).
2.5. Retinal photoisomerase subfamily and peropsin subfamily. Retinochrome and retinal G protein-coupled receptor (RGR), the members of the retinal photoisomerase subfamily, have been identified in the molluscan and vertebrate retinas, specifically in the inner segments of the visual cells (Ozaki et al., 1986; Terakita et al., 1989), and in the retinal pigment epithelium (RPE) (Jiang et al., 1993), respectively. Although most opsins bind to 11-cis-retinal as a chromophore and form photosensitive pigments that act as light-sensing GPCRs, retinochrome and RGR bind to all-trans-retinal as a chromophore (Hara and Hara, 1968; Hao and Fong, 1999). Irradiation of these two pigments causes isomerization of the chromophore to the 11-cis form (Hara and Hara, 1968; Hao and Fong, 1999), suggesting that these opsins enzymatically generate 11-cis-retinal, and supply it to the visual opsins (Terakita et al., 1989; Chen et al., 2001) (Figure 6).
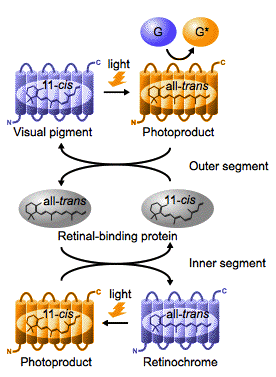
Figure 6. Schematic representation of the molecular function of squid retinochrome in the photoreceptor cell. Squid retinochrome, which is localized in the inner segment of the photoreceptor cell, binds to all-trans-retinal as a chromophore, and light absorption causes trans to cis isomerization of the retinal. The 11-cis- and all-trans-retinals are transported to the outer segment and the inner segment by the retinal-binding protein for regeneration of the visual pigment and retinochrome, respectively. (Modified from Terakita et al., 1989)
As far as we know, there has been no evidence that retinochrome/RGR and/or their photoproducts are coupled to G proteins. Recently, it has also been reported that RGR accelerates the conversion of retinyl esters to 11-cis-retinal, independently of light (Wenzel et al., 2005), and that RGR mediates light-dependent translocation of all-trans-retinyl esters in RPE cells (Radu et al., 2008). The findings suggest that RGR is related to non-photochemical regeneration of 11-cis-retinal in RPE cells. Further study is necessary to elucidate the varied functions of RGR.
Peropsin was first found in the RPE of the mammalian eye (Sun et al., 1997), and identified from non-mammalian vertebrates and invertebrates, amphioxus and spider (Koyanagi et al., 2002; Nagata et al., 2010), but its function remains unknown. Both amphioxus (deuterostome) and spider (protostome) peropsins bind all-trans-retinal as a chromophore, and light isomerizes it to the 11-cis form (Koyanagi et al., 2002; Nagata et al., 2010 ) as retinochrome and RGR, suggesting a possible function of peropsin as a retinal-photoisomerase (Figure 6). Unlike retinochrome and RGR, however, peropsin contains a 'NPXXY' motif, which is highly conserved among most opsins and other GPCRs, and exhibits a bistable nature (Nagata et al., 2010) as Gq-coupled and Go-coupled opsins and parapinopsin as described above. These facts imply that peropsin might act as a light-sensing opsin pigment. Furthermore, there is a significant difference in absorption maximum between amphioxus (~490 nm) and spider peropsin (~535 nm). In addition, a bistable nature has been observed in spider peropsin but not clearly in amphioxus peropsin. The differences suggest that peropsin function in deuterostomes and protostomes might be somewhat different.
2.6. Other opsins: encephalopsin/tmt-opsin and neuropsin. The members of the encephalopsin/tmt-opsin subfamily have been found in a variety of vertebrates and invertebrates, but their molecular function remains unclear. Vertebrates have two subgroups, encephalopsin (or opn3) and teleost multiple tissue opsin (tmt-opsin) (Velarde et al., 2005; Peirson et al., 2009). Encephalopsin has been identified for the first time in humans and mice, and mouse encephalopsin is strongly expressed in the brain and testes, and weakly in other tissues (Blackshaw and Snyder, 1999; Lein et al., 2007). Tmt-opsin is expressed in multiple tissues, i.e., brain, liver and eye, of teleosts (Moutsaki et al., 2003). In invertebrates, encephalopsin has been identified from some species of insects, such as mosquitoes, bees, moths and beetles (Hill et al., 2002; Velarde et al., 2005; Nene et al., 2007), sea urchins (Raible et al., 2006; Ooka et al., 2010), and the annelid worm (Arendt et al., 2004) (insect and annelid worm encephalopsins are also called pteropsin and c-opsin, respectively). Although encephalopsin is reported to be expressed in the brain in the annelid worm and the honeybee (Arendt et al., 2004; Velarde et al., 2005), its detailed expression pattern in varied invertebrates remains largely unknown.
Neuropsin was first identified in mice and humans and is distributed in the eye, brain, testes and spinal cord based on RT-PCR (Tarttelin et al., 2003). Neuropsin is expressed in the developing chicken retina (Tomonari et al., 2008) and in quail deep brain photoreceptors, which may regulate the seasonal cycle of reproduction (Nakane et al., 2010). Xenopus oocytes in which quail neuropsin was heterologously expressed exhibited light-dependent activation of membrane currents, with an action spectrum with a peak at ~420 nm, suggesting quail neuropsin functions as a violet-sensitive pigment (Nakane et al., 2010). These facts suggest that neuropsin functions in detection of seasonal changes of the photoperiod in quails.
3. Molecular Evolution of Vertebrate Visual Pigments from the Viewpoint of Counterion.
As described above, the opsin family is divided into eight subfamilies. Previous spectroscopic studies revealed that six subfamilies contain opsins forming visible-light sensitive pigments with chromophore retinal. For visible light absorption, all opsins contain an essential amino acid residue called "counter ion", in addition to a retinal-binding site, Lys296 (in the bovine rhodopsin numbering system), where chromophore retinal covalently binds through a protonated Schiff base linkage (box inset, Figure 7). The proton on the Schiff base is necessary for visible light absorption, but energetically unstable within the opsin molecule. In opsin pigments, a negatively charged amino acid residue, counterion, stabilizes the protonated Schiff base, and is an essential amino acid residue for opsin pigments to absorb visible light.
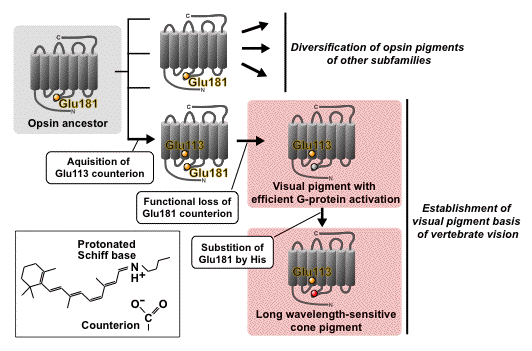
Figure 7. Counterion displacement in the molecular evolution of vertebrate visual and non-visual pigments. Counterion stabilizes the retinylidene protonated Schiff base (box inset). It is suggested that counterion displacement from Glu181 to Glu113 enabled the pigments to acquire efficient G protein activation and sensitivity to long-wavelength light of the cone pigment, leading to the establishment of a molecular basis of vertebrate vision.
The counterion of vertebrate visual pigment rhodopsin has been identified as Glu113 (in the bovine rhodopsin numbering system), in the third transmembrane helix. Glu113 is highly conserved among vertebrate visual and non-visual opsin subfamilies, but not among the members of other subfamilies, i.e., the amino acid residues at position 113 are occupied by methionine and tyrosine in retinochrome, and members of other subfamilies such as Gq-coupled opsin, Go-coupled opsin and peropsin subfamilies. Thus, we compared the functional counterion of the members of different subfamilies to obtain an insight into evolution and diversity of opsins.
A series of retinochrome mutants revealed that Glu181 (in the bovine rhodopsin numbering system) serves as its counterion. Further mutational analyses of other opsin pigments also revealed that Glu181 serves as the counterion in Go-coupled opsin pigment and peropsin. Because Glu181 is highly conserved in members of all opsin subfamilies, it is strongly suggested that vertebrate visual and non-visual pigments (Gt-coupled opsin pigments) contain Glu113 counterion but other opsin pigments possess Glu181 counterion. Recently, the crystal structure of squid rhodopsin, which belongs to the Gq-coupled opsin subfamily, was reported (Murakami and Kouyama, 2008; Shimamura et al., 2008). In the crystal structure, Glu181 is situated ~5 Å away from the Schiff base region. A recent report using hybrid quantum mechanics/molecular dynamics (QM/MM) calculations indicated that Glu181 is negatively charged, and serves as the main counterion of squid rhodopsin without a direct hydrogen bond (Sekharan et al., 2010).
The difference in counterion position may be relevant to the spectroscopic characteristics of the pigments; opsin pigments that have a Glu113 counterion, i.e., vertebrate visual pigments, are characterized as bleaching pigments whose photoproduct is unstable and dissociates its chromophore, whereas almost all opsin pigments containing the Glu181 counterion are bistable pigments, whose photoproduct is stable and reverts to its original dark state by subsequent light absorption (see Figure 4). Interestingly, Glu181 serves as a counterion at least in the photoproduct of vertebrate non-visual pigment parapinopsin, which is phylogenetically close to vertebrate bleaching pigments but has a bistable nature, much like invertebrate Gq-coupled visual pigments, melanopsins and Go-coupled opsin pigment. Taken together, it can be speculated that counterion displacement from Glu181 to Glu113 occurred during the molecular evolution of vertebrate visual and non-visual pigments (Figure 7).
Counterion displacement resulted in two important amino acid residues, a new counterion Glu113 and a former counterion Glu181, each of which potentially mediates new functional properties of vertebrate visual pigments. In contrast to the Glu181 counterion, the Glu113 counterion functions as an intramolecular switch to activate G protein efficiently; that is, the electrostatic interaction between the protonated Schiff base and Glu113 keeps the protein in the inactive form, and the active state could be formed concurrently with the loss of this interaction (protonation of a Glu113 counterion), resulting in a large conformational change of the protein and efficient activation of G protein. Interestingly, G protein activation efficiency of the Go-coupled opsin pigment and parapinopsin are lower than that of bovine rhodopsin by ~50 times and ~20 times, respectively (Terakita et al., 2004). A recent site-specific fluorescence study suggested that the conformational change of the protein moiety of parapinopsin upon photoactivation is smaller than that of bovine rhodopsin (Tsukamoto et al., 2009). These studies strongly suggest that the new counterion could provide a larger conformational change of opsin pigments and higher G protein activation efficiency, which is a physiologically suitable property for a highly photosensitive visual system.
With respect to the former counterion, we can find an interesting example in vertebrate red-sensitive cone visual pigment, where the amino acid residue at position 181 is occupied by histidine. It is well known that His181 is an essential amino acid residue for binding the chloride ion; His181 forms a chloride-binding site with other residues, and binding of chloride is responsible for red-shift of the absorption spectrum. In other words, His181 is an essential amino acid residue for red-sensitivity of the pigment. Thus, we can conclude that the mutation of Glu181 to His181, after acquisition of the new counterion Glu113, could promote the acquisition of red-sensitive cone pigment (Figure 7).
Taken together, counterion displacement may be the first step at a molecular level for vertebrate vision with high photosensitivity, and color vision.
4. Evolutionary Aspects of Diversity of Rhodopsins and Photoreceptor Cells.
As described in Section 2, opsin pigments are phylogenetically diverged, and also functionally varied. Photoreceptor cells, where opsin pigments function, also show variety in terms of morphology and cellular light response. Since not only the spectroscopic characteristics of opsin pigments, but also the type of phototransduction cascade triggered by opsin pigments closely relates to characteristics of photoreceptor cell response, it is quite possible that photoreceptor cells evolved with opsin pigments. In this Section, we focus discussion on the evolution of photoreceptor cells from the functional and phylogenetical viewpoints of opsin pigments.
As seen in rod and cone cells, most photoreceptor cells possess a highly developed membrane portion called the outer segment, which contains highly dense pigments and is a specialized part for light reception (Eakin, 1965). Despite the common feature, the morphology of the photoreceptor cells varies, depending on animals. In the 1970s, photoreceptor cell morphologies were extensively investigated in a wide variety of animals. Based on the morphological divergence among animal photoreceptor cells, Salvini-Plawen and Mayr (1977) argued that animal photoreceptor cells had independently evolved at least 40 times in different groups of animals.
On the other hand, Eakin (1965) hypothesized that animal photoreceptor cells can be classified into the following two groups based on whether the outer segment is derived from a modified cilium or from apical microvilli: ciliary photoreceptor cells and rhabdomeric photoreceptor cells. Interestingly, many deuterostomes, including vertebrates, employ ciliary photoreceptor cells as visual cells, and the rhabdomeric photoreceptor, in contrast, is a predominant type of protostome visual cell. Therefore, it is likely that ciliary photoreceptor cells and rhabdomeric photoreceptor cells evolved in the deuterostome and the protostome lineages, respectively. However, it has been found that some animals, such as scallop (protostome) (Barber et al., 1967) and amphioxus (deuterostome) (Lacalli, 2004), have both ciliary and rhabdomeric photoreceptor cells. These two kinds of photoreceptor cells had already evolved before the protostome and deuterostome split, and both cells exist in both lineages unless secondary loss has occurred (Arendt, 2003; Nilsson, 2005). In this section, we discuss the evolutionary relationship among and between ciliary photoreceptor cells, and rhabdomeric photoreceptor cells, through the animal kingdom, based on several lines of molecular biological evidence.
4.1. Rhabdomeric photoreceptor cell and rhabdomeric photopigment. Rhabdomeric photoreceptor cells are well known as visual cells of protostomes, such as squids and fruit flies. In these cells, opsin pigments that activate Gq-type G protein, namely Gq-coupled opsin pigments, function as visual pigments and trigger inositol phospholipid signaling to generate a depolarizing response of the cell (Terakita et al., 1993; Lee et al., 1994; Yarfitz and Hurley, 1994; Kikkawa et al., 1996; Koyanagi and Terakita, 2008). Rhabdomeric photoreceptor cells are also found in the deuterostome lineage, but underlying opsin pigments are largely unknown. Therefore, it should be verified whether rhabdomeric photoreceptor cells found in both protostome and deuterostome lineages share a common origin.
More interestingly, morphologically identifiable rhabdomeric photoreceptor cells have never been found in vertebrates. The fate of the missing rhabdomeric photoreceptor cell in the vertebrate lineage is a long standing issue. Recently, Arendt (2003) hypothesized that the rhabdomeric photoreceptor cell evolved to be the photosensitive retinal ganglion cell, which recently proved to play roles in circadian photoentrainment and pupillary light response in mammals (Lucas et al., 2001; Berson et al., 2002; Hattar et al., 2003; Lucas et al., 2003; Panda et al., 2003), as some molecules involved in cell differentiation are common among them. In addition, as described above, melanopsin, which was first found in Xenopus melanophore, and is a circadian photopigment in mammalian photosensitive retinal ganglion cells, is similar to visual pigments that exist in protostome rhabdomeric photoreceptor cells at the amino acid sequence level (Provencio et al., 1998; Provencio et al., 2000), suggesting their common origin.
We focused on the cephalochordate amphioxus, aiming to link the protostome rhabdomeric photoreceptor cells, deuterostome rhabdomeric photoreceptor cells and vertebrate photosensitive retinal ganglion cells based on photopigment function, because it is the closest living invertebrate to vertebrates, and it has rhabdomeric photoreceptor cells for putative non-visual functions (Lacalli, 2004). We identified an amphioxus homolog of melanopsin in two types of rhabdomeric photoreceptor cells, the Joseph cells and the photoreceptor cells of dorsal ocelli. We also investigated the biochemical and photochemical properties of amphioxus melanopsin, because photoreceptor properties are directly associated with these characteristics of the photopigment.
Interestingly, the amphioxus melanopsin converts to a thermally stable photoproduct by light absorption, and the stable photoproduct reverts to the original dark state by subsequent light absorption, showing a bistable nature, like protostome Gq-coupled visual pigments in rhabdomeric photoreceptors and unlike vertebrate visual pigments (Koyanagi et al., 2005). Immunohistochemical analyses revealed that melanopsin is colocalized with Gq in the two kinds of rhabdomeric cells, and in vitro biochemical studies showed that amphioxus melanopsin actually activates Gq as efficiently as squid and honeybee visual pigments in a light-dependent manner (Koyanagi et al., 2005; Terakita et al., 2008).
These observations show that melanopsin in deuterostome rhabdomeric photoreceptor cells and visual pigments in protostome rhabdomeric photoreceptor cells possess almost the same molecular properties, and trigger a Gq-mediated phototransduction cascade. This finding was confirmed by a recent electrophysiological study that amphioxus melanopsin-expressing cells exhibit light-dependent depolarizing responses like protostome visual cells (Gomez et al., 2009). Therefore, it can be concluded that photopigments functioning in protostome and deuterostome rhabdomeric photoreceptor cells, and vertebrate photosensitive retinal ganglion cells are evolutionary and functionally equivalent.
This conclusion also bridges the evolutionary gap among these photoreceptor cells in the following manner; in the chordate lineage, rhabdomeric photoreceptor cells with a Gq-mediated phototransduction cascade were evidently excluded from the retina portion concerned with image-forming vision, and were relegated instead to a non-visual role, as they apparently also are in the amphioxus. Subsequently, in the vertebrate lineage, these cells must have lost their rhabdomeric morphology, resulting in the emergence of a photosensitive retinal ganglion cell (Koyanagi et al., 2005; Koyanagi and Terakita, 2008).
4.2. Ciliary photoreceptor cell and ciliary photopigment. As described above, ciliary photoreceptors, distinguishable from rhabdomeric photoreceptor cells by the presence of modified cilia at the base of outer segments, are well known as vertebrate rods and cones, and they are also found in several invertebrates (Terakita, 2005) (Figure 5). In contrast to the rhabdomeric photoreceptor cells, several types of opsins and phototransduction cascades have been found in ciliary photoreceptor cells thus far. In vertebrate rods and cones, Gt-coupled opsin pigment triggers the Gt-mediated phototransduction cascade, causing a decrease of intracellular cGMP concentration to close cyclic nucleotide-gated (CNG) channels (Kuhn, 1980; Stryer, 1986; Yau and Baylor, 1989) (see Figure 3). Encephalopsin or c-opsin, which is related to Gt-coupled opsin, is also found in the ciliary cells in the marine ragworm brain (Arendt et al., 2004). Go-coupled opsin pigment, which is phylogenetically distinct from Gt-coupled opsin, is coupled to Go, causing elevation of cGMP as a second messenger in scallop ciliary visual cells (Kojima et al., 1997; Gomez and Nasi, 2000). Interestingly, parietopsin, which is closely related to Gt-coupled opsin but not to Go-coupled opsin, was also shown to be coupled to Go in the ciliary photoreceptor cells of lizard parietal eyes (Su et al., 2006).
Recently, we identified the visual pigment and phototransduction cascade in the ciliary-type visual cells of pre-bilaterian animals, which emerged before the radiation of protostomes and deuterostomes, to understand the varied ciliary-type phototransduction cascades phylogenetically (Koyanagi et al., 2008b). We isolated the opsin gene from box-jellyfish, which are known to have ciliary visual cells in well-developed camera-type eyes. The spectroscopic analysis of the jellyfish opsin pigment obtained by expression in cultured cells and reconstitution with 11-cis-retinal showed that the pigment functions as a green-sensitive pigment with absorption maximum at ~500 nm in the ciliary visual cells. Interestingly, immunohistochemical analyses clearly showed that the jellyfish visual pigment colocalized with Gs, not Gq, Gt, or Go in the visual cells. We also demonstrated that light-dependent cAMP increase takes place in jellyfish visual cells. A series of evidence revealed that the ciliary photoreceptor in pre-bilaterian jellyfish eyes employs the Gs-mediated phototransduction cascade, indicating that there are three kinds of cascades, Gt, Go and Gs in ciliary photoreceptors of different animals (Koyanagi et al., 2008b).
Accordingly, judging from the G protein subtype that mediates light signaling, the ciliary photoreceptor cells seem to have polyphyletic origins. However, despite these differences, all of these cells employ cyclic nucleotides (cGMP or cAMP) as the second messenger in the phototransduction cascade. In the Gt-mediated phototransduction cascade of vertebrate rods and cones, CNG (cyclic nucleotide-gated) channels are used to generate cellular responses (Yau and Baylor, 1989; Kaupp and Seifert, 2002). Similarly, in the Go-mediated phototransduction of molluscan photoreceptor cells, the function of CNG channels to generate cellular responses was inferred from pharmacological experiments (Gotow et al., 1994; Gomez and Nasi, 1997). In addition, we found a cDNA encoding a channel, which fell into the CNG subfamily including vertebrate rod and cone CNGs, from box jellyfish eyes (Koyanagi et al., 2008b). These observations, together with the fact that all known CNGs respond to both cAMP and cGMP (Kaupp and Seifert, 2002), suggest a monophyletic group of animal phototransduction cascades characterized by employing cyclic nucleotides, presenting an evolutionary linkage from prebilaterian phototransduction to vertebrate phototransduction.
Surprisingly, the Gs-mediated phototransduction cascade in jellyfish visual cells exhibits overall similarities with the vertebrate olfactory signaling cascade, which is composed of a Gs-type G protein, olfactory neuron-specific G protein (Golf) and adenylyl cyclase type III, and elicits increases in cAMP, and activation of CNG channels belonging to the same group as rod and cone CNGs (Firestein, 2001) and jellyfish CNG channel. Furthermore, the vertebrate olfactory sensory neuron has ciliary morphology. Therefore, it may be an intriguing hypothesis that the vertebrate olfactory sensory neuron shares an evolutionarily common origin with the ciliary photoreceptor cells. This hypothesis could explain the fact that the receptor origin and the signaling cascade of vertebrate olfactory system are far different from those of the insect olfactory system, in which receptors themselves function as ion channels (Bargmann, 2006; Sato et al., 2008; Wicher et al., 2008).
4.3. Classification and origin of animal photoreceptor cell and photopigment. Based on several lines of evidence described above, we propose a classification of varied animal photoreceptor cells, the rhabdomeric photoreceptor cell containing phosphoinositol signaling mediated by Gq, and the ciliary photoreceptor cell containing cyclic nucleotide signaling mediated by Gt, by Go, or by Gs (Figure 3, Figure 5). Classification based on the second messenger, which is coupled with the channel to generate an electrical response of photoreceptor cells, is quite reasonable from a functional viewpoint because for photoreceptor cell function, the input (receptor) and output (channel) must be primarily important, and each component that underlies the signaling cascade could be secondary.
There are still several unresolved issues, including what kind of photoreceptor cells and what kind of phototransduction cascades are ancestral. As for the ancestral photoreceptor cell type, Arendt et al. (2009). recently provided an intriguing hypothesis called the 'division of labor' model of eye evolution that the ancestral photoreceptor cell was multifunctional with rhabdomeric structures for photoreception, pigment granules for light shading, and locomotor cilia for steering. Then, the functional segregation of the ancestral photoreceptor cell occurred to eventually evolve into the photoreceptor cell with rhabdomeric structures as well as cilia with/without pigment granules. This hypothesis is supported by the fact that there are multifunctional photoreceptor cells in many animal lineages including lower animals. For example, the planula larvae of a box jellyfish (Tripedalia cystophora) possess photoreceptor cells with rhabdomeric structures, pigment granules and cilia (Nordstrom et al., 2003), and the photoreceptor cells in a sponge larvae have pigment granules as well as cilia for locomotion (Leys and Degnan, 2001).
As for the ancestral phototransduction cascade, we here provide one reasonable hypothesis, which has arisen from our recent finding that Gs-mediated cAMP signalling is used in the lower animal. A yeast homologue of G protein (Gpa2) causes an increase in cAMP via adenylyl cyclase (Ivey and Hoffman, 2005), strongly suggesting that the ancestral G protein in animal cells functions to increase cAMP. Accordingly, the cell in which the G-protein-mediated cAMP signaling was coupled to an already-existing CNG channel and a newly-evolved opsin pigment could be the first photoreceptor cell of animals if the emergence of animal photoreceptor cells antedated the divergence of G protein in the animal lineage. Thereafter, during the course of animal evolution, signal transduction cascades could have diverged and been reconstructed under the constraint that CNG channels are used for generating cellular responses. Further investigation on the opsin pigment and phototransduction cascade in such lower animals may provide clues to the ancestral animal phototransduction cascade.
5. Conclusion
More than 2000 kinds of opsins have been identified thus far, and the opsin family is divided into several subfamilies. We have found new opsin-based pigments, Go-coupled and Gs-coupled opsin-based pigments, in addition to well known opsin-based pigments that drive Gt- and Gq-mediated cascades, respectively. Based on the second messengers, which open and close channels, a classification of varied animal photoreceptor cells is proposed, phosphoinositol signaling mediated by Gq in rhabdomeric photoreceptor cells, and cyclic nucleotide signaling mediated by Gt, by Go, or by Gs in the ciliary photoreceptor cells. Further understanding of evolutionary bridge between these phototransduction cascades and photoreceptor cell morphology, as well as eye structures (Lamb et al., 2009; Nilsson, 2009), could provide a scenario about evolution and the diversity of animal photoreception.
References
Alvarez CE (2008) On the origins of arrestin and rhodopsin. BMC Evol Biol 8:222.
Arendt D (2003) Evolution of eyes and photoreceptor cell types. Int J Dev Biol 47:563-71.
Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J (2004) Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 306:869-71.
Arendt D, Hausen H, Purschke G (2009) The 'division of labour' model of eye evolution. Philos Trans R Soc Lond B Biol Sci 364:2809-17.
Barber VC, Evans EM, Land MF (1967) The fine structure of the eye of the mollusc Pecten maximus. Cell Tiss Res 76:295-312.
Bargmann CI (2006) Comparative chemosensation from receptors to ecology. Nature 444:295-301.
Barnard AR, Hattar S, Hankins MW, Lucas RJ (2006) Melanopsin regulates visual processing in the mouse retina. Curr Biol 16:389-95.
Bellingham J, Chaurasia SS, Melyan Z, Liu C, Cameron MA, Tarttelin EE, Iuvone PM, Hankins MW, Tosini G, Lucas RJ (2006) Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol 4:e254.
Berson DM, Dunn FA, Takao M (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070-3.
Berson DM (2007) Phototransduction in ganglion-cell photoreceptors. Pflugers Arch 454:849-55.
Blackshaw S, Snyder SH (1997) Parapinopsin, a novel catfish opsin localized to the parapineal organ, defines a new gene family. J Neurosci 17:8083-92
Blackshaw S, Snyder SH (1999) Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci 19:3681-90.
Chen P, Hao W, Rife L, Wang XP, Shen D, Chen J, Ogden T, Van Boemel GB, Wu L, Yang M et al. (2001) A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet 28:256-60.
Contin MA, Verra DM, Guido ME (2006) An invertebrate-like phototransduction cascade mediates light detection in the chicken retinal ganglion cells. FASEB J 20:2648-50.
Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD (2005) Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433:749-54.
Downes GB, Gautam N (1999) The G protein subunit gene families. Genomics 62:544-52.
Eakin RM (1965) Evolution of photoreceptors. Cold Spring Harb Symp Quant Biol 30:363-70.
Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S (2010) Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 67:49-60.
Engels A, Reichert H, Gehring WJ, Gartner W (2000) Functional expression of a locust visual pigment in transgenic Drosophila melanogaster. Eur J Biochem 267:1917-22.
Farrens DL, Khorana HG (1995) Structure and function in rhodopsin. Measurement of the rate of metarhodopsin II decay by fluorescence spectroscopy. J Biol Chem 270:5073-6.
Feiler R, Bjornson R, Kirschfeld K, Mismer D, Rubin GM, Smith DP, Socolich M, Zuker CS (1992) Ectopic expression of ultraviolet-rhodopsins in the blue photoreceptor cells of Drosophila - visual physiology and photochemistry of transgenic animals. J Neurosci 12:3862-8.
Firestein S (2001) How the olfactory system makes sense of scents. Nature 413:211-8.
Fu Y, Zhong H, Wang MH, Luo DG, Liao HW, Maeda H, Hattar S, Frishman LJ, Yau KW (2005) Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Nat Acad Sci USA 102:10339-44.
Gomez M, Nasi E (1997) Antagonists of the cGMP-gated conductance of vertebrate rods block the photocurrent in scallop ciliary photoreceptors. J Physiol 500 ( Pt 2):367-78.
Gomez M, Nasi E (2000) Light transduction in invertebrate hyperpolarizing photoreceptors: possible involvement of a Go-regulated guanylate cyclase. J Neurosci 20:5254-63.
Gomez M, Angueyra JM, Nasi E (2009) Light-transduction in melanopsin-expressing photoreceptors of Amphioxus. Proc Nat Acad Sci USA 106:9081-6.
Gotow T, Nishi T, Kijima H (1994) Single K+ channels closed by light and opened by cyclic GMP in molluscan extra-ocular photoreceptor cells. Brain Res 662:268-72.
Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM (2008) Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol 99:2522-32.
Halford S, Pires SS, Turton M, Zheng L, Gonzalez-Menendez I, Davies WL, Peirson SN, Garcia-Fernandez JM, Hankins MW, Foster RG (2009) VA opsin-based photoreceptors in the hypothalamus of birds. Curr Biol 19:1396-402.
Hao W, Fong HK (1999) The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium. J Biol Chem 274:6085-90.
Hara T, Hara R (1968) Regeneration of squid retinochrome. Nature 219:450-4.
Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG et al. (2003) Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424:76-81.
Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ (2002) G protein-coupled receptors in Anopheles gambiae. Science 298:176-8
Hisatomi O, Tokunaga F (2002) Molecular evolution of proteins involved in vertebrate phototransduction. Comp Biochem Physiol B Biochem Mol Biol 133:509-22.
Isoldi MC, Rollag MD, Castrucci AM, Provencio I (2005) Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc Nat Acad Sci USA 102:1217-21.
Ivey FD, Hoffman CS (2005) Direct activation of fission yeast adenylate cyclase by the Gpa2 G
 of the glucose signaling pathway. Proc Nat Acad Sci USA 102:6108-13.
of the glucose signaling pathway. Proc Nat Acad Sci USA 102:6108-13.
Jiang M, Pandey S, Fong HK (1993) An opsin homologue in the retina and pigment epithelium. Invest Ophthalmol Vis Sci 34:3669-78.
Kasahara T, Okano T, Haga T, Fukada Y (2002) Opsin-G11-mediated signaling pathway for photic entrainment of the chicken pineal circadian clock. J Neurosci 22:7321-5.
Kaupp UB, Seifert R (2002) Cyclic nucleotide-gated ion channels. Physiol Rev 82:769-824.
Kawano-Yamashita E, Terakita A, Koyanagi M, Shichida Y, Oishi T, Tamotsu S (2007) Immunohistochemical characterization of a parapinopsin-containing photoreceptor cell involved in the ultraviolet/green discrimination in the pineal organ of the river lamprey Lethenteron japonicum. J Exp Biol 210:3821-9.
Kikkawa S, Tominaga K, Nakagawa M, Iwasa T, Tsuda M (1996) Simple purification and functional reconstitution of octopus photoreceptor Gq, which couples rhodopsin to phospholipase C. Biochemistry 35:15857-64.
Kojima D, Terakita A, Ishikawa T, Tsukahara Y, Maeda A, Shichida Y (1997) A novel Go-mediated phototransduction cascade in scallop visual cells. J Biol Chem 272:22979-82.
Kojima D, Mano H, Fukada Y (2000) Vertebrate ancient-long opsin: a green-sensitive photoreceptive molecule present in zebrafish deep brain and retinal horizontal cells. J Neurosci 20:2845-51.
Koyanagi M, Terakita A, Kubokawa K, Shichida Y (2002) Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis- and all-trans-retinals as their chromophores. FEBS Lett 531:525-8.
Koyanagi M, Kawano E, Kinugawa Y, Oishi T, Shichida Y, Tamotsu S, Terakita A (2004) Bistable UV pigment in the lamprey pineal. Proc Nat Acad Sci USA 101:6687-91.
Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A (2005) Cephalochordate melanopsin: Evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol 15:1065-9.
Koyanagi M, Nagata T, Katoh K, Yamashita S, Tokunaga F (2008a) Molecular evolution of arthropod color vision deduced from multiple opsin genes of jumping spiders. J Mol Evol 66:130-7.
Koyanagi M, Takano K, Tsukamoto H, Ohtsu K, Tokunaga F, Terakita A (2008b) Jellyfish vision starts with cAMP signaling mediated by opsin-Gs cascade. Proc Natl Acad Sci USA 105:15576-80.
Koyanagi M, Terakita A (2008) Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem Photobiol 84:1024-30.
Kozmik Z, Ruzickova J, Jonasova K, Matsumoto Y, Vopalensky P, Kozmikova I, Strnad H, Kawamura S, Piatigorsky J, Paces V et al. (2008) Assembly of the cnidarian camera-type eye from vertebrate-like components. Proc Nat Acad Sci USA 105:8989-93.
Kuhn H (1980) Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature 283:587-9.
Lacalli TC (2004) Sensory systems in amphioxus: a window on the ancestral chordate condition. Brain Behav Evol 64:148-62.
Lamb TD, Arendt D, Collin SP (2009) The evolution of phototransduction and eyes. Philos Trans R Soc Lond B Biol Sci 364:2791-3.
Lee YJ, Shah S, Suzuki E, Zars T, O'Day PM, Hyde DR (1994) The Drosophila dgq gene encodes a G
 protein that mediates phototransduction. Neuron 13:1143-57.
protein that mediates phototransduction. Neuron 13:1143-57.
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ et al. (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168-76.
Leys SP, Degnan BM (2001) Cytological basis of photoresponsive behavior in a sponge larva. Biol Bull 201:323-38.
Lucas RJ, Douglas RH, Foster RG (2001) Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci 4:621-6.
Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW (2003) Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 299:245-7.
Matthews RG, Hubbard R, Brown PK, Wald G (1963) Tautomeric Forms of Metarhodopsin. J Gen Physiol 47:215-40.
Max M, McKinnon PJ, Seidenman KJ, Barrett RK, Applebury ML, Takahashi JS, Margolskee RF (1995) Pineal opsin: a nonvisual opsin expressed in chick pineal. Science 267:1502-6.
Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW (2005) Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433:741-5.
Moutsaki P, Whitmore D, Bellingham J, Sakamoto K, David-Gray ZK, Foster RG (2003) Teleost multiple tissue (tmt) opsin: a candidate photopigment regulating the peripheral clocks of zebrafish? Brain Res Mol Brain Res 112:135-45.
Murakami M, Kouyama T (2008) Crystal structure of squid rhodopsin. Nature 453:363-7.
Mure LS, Rieux C, Hattar S, Cooper HM (2007) Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms 22:411-24.
Mure LS, Cornut PL, Rieux C, Drouyer E, Denis P, Gronfier C, Cooper HM (2009) Melanopsin bistability: a fly's eye technology in the human retina. PLoS One 4:e5991.
Musio C and Santillo S (2009) Non-visual photoreception in invertebrates, on Photobiological Sciences Online (KC Smith, ed.) American Society for Photobiology, http://www.photobiology.info/
Nagata T, Koyanagi M, Tsukamoto H, Terakita A (2010) Identification and characterization of a protostome homologue of peropsin from a jumping spider. J Comp Physiol A 196:51-9.
Nakamura A, Kojima D, Imai H, Terakita A, Okano T, Shichida Y, Fukada Y (1999) Chimeric nature of pinopsin between rod and cone visual pigments. Biochemistry 38:14738-45.
Nakane Y, Ikegami K, Ono H, Yamamoto N, Yoshida S, Hirunagi K, Ebihara S, Kubo Y, Yoshimura T (2010) A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Nat Acad Sci USA 107:15264-8.
Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M et al. (2007) Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316:1718-23.
Nilsson DE (2005) Photoreceptor evolution: ancient siblings serve different tasks. Curr Biol 15:R94-6.
Nilsson DE (2009) The evolution of eyes and visually guided behaviour. Philos Trans R Soc Lond B Biol Sci 364:2833-47.
Nordstrom K, Wallen R, Seymour J, Nilsson D (2003) A simple visual system without neurons in jellyfish larvae. Proc Biol Sci 270:2349-54.
Okano T, Yoshizawa T, Fukada Y (1994) Pinopsin is a chicken pineal photoreceptive molecule. Nature 372:94-7.
Ooka S, Katow T, Yaguchi S, Yaguchi J, Katow H (2010) Spatiotemporal expression pattern of an encephalopsin orthologue of the sea urchin Hemicentrotus pulcherrimus during early development, and its potential role in larval vertical migration. Dev Growth Differ 52:195-207.
Ozaki K, Terakita A, Hara R, Hara T (1986) Rhodopsin and retinochrome in the retina of a marine gastropod, Conomulex luhuanus. Vision Res 26:691-705.
Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE et al. (2000) Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289:739-45.
Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T (2005) Illumination of the melanopsin signaling pathway. Science 307:600-4.
Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M et al. (2003) Melanopsin is required for non-image-forming photic responses in blind mice. Science 301:525-7.
Peirson SN, Halford S, Foster RG (2009) The evolution of irradiance detection: melanopsin and the non-visual opsins. Philos Trans R Soc Lond B Biol Sci 364:2849-65.
Plachetzki DC, Degnan BM, Oakley TH (2007) The origins of novel protein interactions during animal opsin evolution. PLoS One 2:e1054.
Plachetzki DC, Fong CR, Oakley TH (2010) The evolution of phototransduction from an ancestral cyclic nucleotide gated pathway. Proc Biol Sci 277:1963-9.
Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD (1998) Melanopsin: An opsin in melanophores, brain, and eye. Proc Nat Acad Sci USA 95:340-5.
Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD (2000) A novel human opsin in the inner retina. J Neurosci 20:600-5.
Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM (2005) Induction of photosensitivity by heterologous expression of melanopsin. Nature 433:745-9.
Radu RA, Hu J, Peng J, Bok D, Mata NL, Travis GH (2008) Retinal pigment epithelium-retinal G protein receptor-opsin mediates light-dependent translocation of all-trans-retinyl esters for synthesis of visual chromophore in retinal pigment epithelial cells. J Biol Chem 283:19730-8.
Raible F, Tessmar-Raible K, Arboleda E, Kaller T, Bork P, Arendt D, Arnone MI (2006) Opsins and clusters of sensory G-protein-coupled receptors in the sea urchin genome. Dev Biol 300:461-75.
Salvini-Plawen L, Mayr E (1977) On the evolution of photoreceptors and eyes. Evol Biol 10:207-263.
Sato K, Pellegrino M, Nakagawa T, Vosshall LB, Touhara K (2008) Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452:1002-6.
Sekharan S, Altun A, Morokuma K (2010) Photochemistry of visual pigment in a G(q) protein-coupled receptor (GPCR)--insights from structural and spectral tuning studies on squid rhodopsin. Chemistry 16:1744-9.
Shichida Y, Imai H (1998) Visual pigment: G-protein-coupled receptor for light signals. Cell Mol Life Sci 54:1299-315.
Shichida Y, Matsuyama T (2009) Evolution of opsins and phototransduction. Philos Trans R Soc Lond B Biol Sci 364:2881-95.
Shimamura T, Hiraki K, Takahashi N, Hori T, Ago H, Masuda K, Takio K, Ishiguro M, Miyano M (2008) Crystal structure of squid rhodopsin with intracellularly extended cytoplasmic region. J Biol Chem 283:17753-6.
Simon MI, Strathmann MP, Gautam N (1991) Diversity of G proteins in signal transduction. Science 252:802-8.
Solessio E, Engbretson GA (1993) Antagonistic chromatic mechanisms in photoreceptors of the parietal eye of lizards. Nature 364:442-5
Soni BG, Foster RG (1997) A novel and ancient vertebrate opsin. FEBS Lett 406:279-83.
Soni BG, Philp AR, Foster RG, Knox BE (1998) Novel retinal photoreceptors. Nature 394:27-8.
Stryer L (1986) Cyclic GMP cascade of vision. Annu Rev Neurosci 9:87-119.
Su CY, Luo DG, Terakita A, Shichida Y, Liao HW, Kazmi MA, Sakmar TP, Yau KW (2006) Parietal-eye phototransduction components and their potential evolutionary implications. Science 311:1617-21.
Suga H, Schmid V, Gehring WJ (2008) Evolution and functional diversity of jellyfish opsins. Curr Biol 18:51-5.
Sun H, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J (1997) Peropsin, a novel visual pigment-like protein located in the apical microvilli of the retinal pigment epithelium. Proc Nat Acad Sci USA 94:9893-8.
Taddei-Ferretti C, Musio C (2000) Photobehaviour of Hydra (Cnidaria, Hydrozoa) and correlated mechanisms: a case of extraocular photosensitivity. J Photochem Photobiol B 55:88-101.
Taniguchi Y, Hisatomi O, Yoshida M, Tokunaga F (2001) Pinopsin expressed in the retinal photoreceptors of a diurnal gecko. FEBS Lett 496:69-74.
Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ (2003) Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett 554:410-6.
Terakita A, Hara R, Hara T (1989) Retinal-binding protein as a shuttle for retinal in the rhodopsin retinochrome system of the squid visual cells. Vision Res 29:639-52.
Terakita A, Hariyama T, Tsukahara Y, Katsukura Y, Tashiro H (1993) Interaction of GTP-binding protein Gq with photoactivated rhodopsin in the photoreceptor membranes of crayfish. FEBS Lett 330:197-200.
Terakita A, Yamashita T, Tachibanaki S, Shichida Y (1998) Selective activation of G-protein subtypes by vertebrate and invertebrate rhodopsins. FEBS Lett 439:110-4.
Terakita A, Yamashita T, Nimbari N, Kojima D, Shichida Y (2002) Functional interaction between bovine rhodopsin and G protein transducin. J Biol Chem 277:40-6.
Terakita A, Koyanagi M, Tsukamoto H, Yamashita T, Miyata T, Shichida Y (2004) Counterion displacement in the molecular evolution of the rhodopsin family. Nat Struct Mol Biol 11:284-9.
Terakita A (2005) The opsins. Genome Biol 6:213.
Terakita A, Tsukamoto H, Koyanagi M, Sugahara M, Yamashita T, Shichida Y (2008) Expression and comparative characterization of Gq-coupled invertebrate visual pigments and melanopsin. J Neurochem 105:883-90.
Tomonari S, Migita K, Takagi A, Noji S, Ohuchi H (2008) Expression patterns of the opsin 5-related genes in the developing chicken retina. Dev Dyn 237:1910-22.
Townson SM, Chang BSW, Salcedo E, Chadwell LV, Pierce NE, Britt SG (1998) Honeybee blue-and ultraviolet-sensitive opsins: Cloning, heterologous expression in Drosophila, and physiological characterization. J Neurosci 18:2412-22.
Tsukamoto H, Terakita A, Shichida Y (2005) A rhodopsin exhibiting binding ability to agonist all-trans-retinal. Proc Nat Acad Sci USA 102:6303-8.
Tsukamoto H, Farrens DL, Koyanagi M, Terakita A (2009) The magnitude of the light-induced conformational change in different rhodopsins correlates with their ability to activate G proteins. J Biol Chem 284:20676-83.
Tsukamoto H, Terakita A (2010) Diversity and functional properties of bistable pigments. Photochem Photobiol Sci, in press.
Velarde RA, Sauer CD, Walden KK, Fahrbach SE, Robertson HM (2005) Pteropsin: a vertebrate-like non-visual opsin expressed in the honey bee brain. Insect Biochem Mol Biol 35:1367-77.
Wald G (1968) Molecular basis of visual excitation. Science 162:230-9.
Wenzel A, Oberhauser V, Pugh EN, Jr., Lamb TD, Grimm C, Samardzija M, Fahl E, Seeliger MW, Reme CE, von Lintig J (2005) The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J Biol Chem 280:29874-84.
Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS (2008) Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452:1007-11.
Yamashita T, Terakita A, Shichida Y (2000) Distinct roles of the second and third cytoplasmic loops of bovine rhodopsin in G protein activation. J Biol Chem 275:34272-9.
Yarfitz S, Hurley JB (1994) Transduction mechanisms of vertebrate and invertebrate photoreceptors. J Biol Chem 269:14329-32.
Yau KW, Baylor DA (1989) Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci 12:289-327.
Yau KW, Hardie RC (2009) Phototransduction motifs and variations. Cell 139:246-64.
Yokoyama S (1997) Molecular genetic basis of adaptive selection: examples from color vision in vertebrates. Annu Rev Genet 31:315-36.
Zuker CS, Mismer D, Hardy R, Rubin GM (1988) Ectopic expression of a minor Drosophila opsin in the major photoreceptor cell class - distinguishing the role of primary receptor and cellular context. Cell 53:475-82.
10/20/10