PHOTOPROTECTION of PLANTS via OPTICAL SCREENING
Alexei Solovchenko
Department of Biophysics
Faculty of Biology, Moscow State University
119991, GSP-1 Moscow, Russia
wundy@mail.ru
Optical Screening and it's Importance for Plant Photoprotection
Plants as photoautotrophic organisms cannot exist without solar radiation energy. On the other hand, photosynthesis proceeds with an optimal rate only within a narrow irradiance range, which is often lower than fluxes of solar radiation reaching plants under natural conditions. Therefore, the light energy absorbed by the photosynthetic apparatus often could not be utilized completely in the course of photochemical reactions. The imbalance between the amount of the absorbed light energy and the plant's capacity for its utilization occurs under high fluxes of solar radiation and/or even under moderate irradiance combined with stresses of different nature, such as extreme temperatures, drought or mineral nutrition deficiencies. There are also other situations when plants are rendered sensitive to damage by excessive fluxes of solar radiation. Thus, in juvenile and senescing plants, the regulation of photosynthetic apparatus functioning is not so perfect in comparison with mature leaves, making them less efficient in the utilization of the absorbed light, and therefore, prone to photodamage by radiation fluxes that usually do not harm mature plants.
Photodamage to photoautotrophic organisms under unfavorable environmental conditions proceeds primarily via the increased generation of reactive oxygen species (ROS), photosensitized in the cells by chlorophylls and a number of endogenous photosensitizers such as porphyrins, flavins, and pterins. Apart from excessive photosynthetically active radiation (PAR), photodamage to plants can be induced by ultraviolet (UV) radiation (Figure 1).
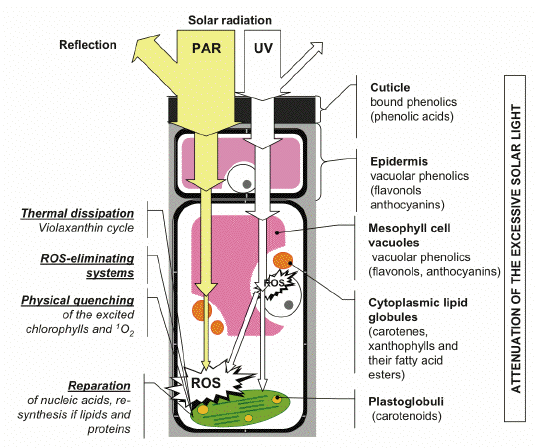
Figure 1. Optical screening is an integral part of a system of photoprotective mechanisms in plants. Under unfavorable environmental conditions, and in situations when the regulation of photosynthesis is impaired, high fluxes of solar radiation induce direct or indirect ROS-mediated damages to plants. Certain mechanisms are responsible for a decrease in ROS levels in the cell and cope with the consequences of photodamage. The screening pigments attenuate the incident radiation, thereby removing, to a considerable extent, the cause of photodamage (harmful UV and excessively absorbed visible quanta). [Reproduced from Solovchenko and Merzlyak, 2008. With kind permission from Springer Science+Business Media.]
It is important to realize that the photoprotective mechanisms based on ROS elimination (Asada, 2006), and the thermal dissipation of the excessive absorbed light energy (Demmig-Adams and Adams, 2006), have certain aspects in common. All of them predominantly cope with the consequences of photodamage by UV and PAR, i.e., by the repair of damaged macromolecules, and the elimination of ROS, and products of their reactions (such as toxic organic peroxides and radical species; see Asada, 2006) already formed in the cell. The efficient operation of these mechanisms requires sufficient levels of energy-rich and/or reducing compounds, which are necessary for repairing DNA, re-synthesis of the membrane lipids and proteins, as well as for the regeneration of important low-molecular antioxidants, such as reduced glutathione and ascorbate.
Over the last two decades, the concept of photoprotective mechanisms based on attenuation or 'passive' optical screening of harmful radiation by extrathylakoid pigments has evolved and became widespread. The ability of plants to respond to strong irradiation by the synthesis and accumulation, within different cell compartments and tissue structures, of the compounds selectively absorbing in the UV or the visible part of the spectrum is the foundation of these mechanisms. In higher plants, these compounds are concentrated in the superficial structures, such as cuticle or epidermis, and/or distributed within cells and tissues. These mechanisms are distinct from the 'classic' or 'active' photoprotective systems listed in the previous paragraph in a number of ways (Figure 1; see also Solovchenko and Merzlyak, 2008). Primarily, they prevent photodamage by alleviating its cause, i.e., the excessive absorption of radiation by the photosynthetic apparatus (PSA), and other photosensitive cell components.
Recent evidence suggests that plant screening pigments possess high photostability both in vitro and in planta. Therefore, a photoprotective screen, once formed, could be maintained with minimal expenditure of energy and valuable metabolites, therefore providing a reliable long-term protection against photodamage. It is important, therefore, that the efficiency of the 'passive' screening of radiation is far less affected by environmental stresses (such as extreme temperatures or drought), which suppress photosynthesis and could impair the ability of the enzymatic systems to provide an adequate level of photoprotection.
At the same time, the initial buildup of photoprotective compounds demands a considerable amount of photoassimilates, such as NADPH and sugars, and energy (in the form of ATP) to be invested in the biosynthesis of screening pigments. The induction of synthesis and accumulation of the pigments in amounts sufficient for accomplishing their photoprotective function (as well as decomposition of earlier accumulated screening compounds) is a relatively slow process, which occurs on the timescale of hours and days. Due to these circumstances, the screening-based mechanisms are warrantable mostly under prolonged action of a stressor; hence, they are of high importance for the long-term adaptation of plants.
Is it Really a Screening Pigment?
The question of specificity is of crucial importance for the discussion of the potential photoprotective function of a plant pigment. This is especially true in the case of compounds that play multifaceted roles in plant organisms, such as phenolics or carotenoids. The latter, for example, participate in different photoprotective mechanisms, including elimination of ROS or dissipation of excessive energy absorbed by chlorophylls. Indeed, a change in metabolism and pigment composition indirectly increasing the resistance of plants to high fluxes of radiation does not necessarily represent a specific high-light response. In particular, numerous responses of plants to spectral quality of radiation are mediated by phyto- and cryptochrome photoreceptors, which induce various biochemical and photomorphogenic effects, including biosynthesis of certain phenolic compounds, which are not necessarily screening-related. Generally, obtaining the solid evidence of participation of certain substances in photoprotection via screening of radiation is complicated, because many constituents of plant cell, apart from mycosporin-like amino acids (MAA) or phenolics, absorb in the UV and visible parts of the spectrum, but serve no specific photoprotective function. For example, the structural phenylpropanoids and lignins comprising cell wall, as well as the condensed aromatic compounds of plant cuticle, strongly absorb in UV, especially in the UV-B range.
It is generally accepted that the compliance with several criteria is necessary to consider a certain compound as photoprotective (screening) pigment (Cockell and Knowland, 1999):
i) This compound should strongly absorb radiation in the spectral band(s) overlapping with the absorption band(s) of the photosynthetic pigments, endogenous photosensitizers, and/or photosensitive components (such as nucleic acids and/or proteins) of the cell.
ii) The irradiation in the corresponding spectral range should trigger the synthesis of the pigment in the natural and model systems (e.g., cell or tissue cultures).
iii) The accumulation of the compound in question should induce the resistance to the radiation in the spectral range of the pigment absorption.
Only the compliance with all of the criteria listed above might be considered as a solid evidence of the participation of certain compound in the protection against photodamage via radiation screening. In addition, the similarity of the action spectrum of the induction of synthesis of a compound and its absorption spectrum could also represent an evidence for participation of this compound in photoprotection via radiation screening, i.e., the irradiation in the band of the absorption maximum of a screening compound should induce its biosynthesis most efficiently. The mutants deficient on the synthesis of different photoprotective pigments are another useful tool for elucidation and proving specificity of screening pigment function in plants.
The Diversity of Screening Pigments
Screening pigments discovered in photoautotrophs including microalgae and higher plants could be roughly divided into four principal groups: MAA, phenolic compounds with key sub-groups of phenylpropanoids, flavonols and anthocyanins, betalains and carotenoids.
Mycosporin-like amino acids (MAA). Many more primitive photoautotrophs including cyanobacteria, red and green microalgae, as well as dinoflagellates, accumulate MAA, the compounds resembling water-soluble mycosporines initially discovered in fungi (Figure 2).
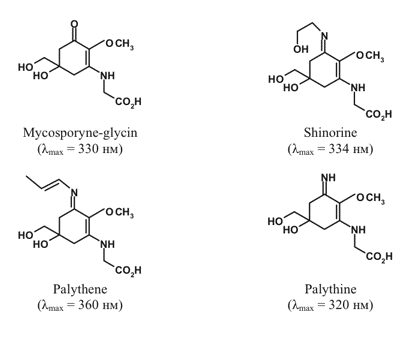
Figure 2. Selected mycosporin-like amino acids and their absorption maxima.
MAA have molar extinction coefficients in the range 24-50 mM-1 cm-1. The abovementioned properties together with high photostability both in vitro and in vivo make MAA efficient UV-screening compounds. Additional details on MAA biosynthesis, natural occurrence and functions can be found in a comprehensive review by Shick and Dunlap (2002); see also the module on Ultraviolet Effects on Phytoplankton, and Sinha and Hder (2007).
Phenolic compounds are amazingly ubiquitous in nature. They are found in every plant species studied so far; more than 20,000 phenolic species are known to date, and most of them were discovered in plants. These compounds are characterized by an extreme diversity of chemical structure (Figure 3).
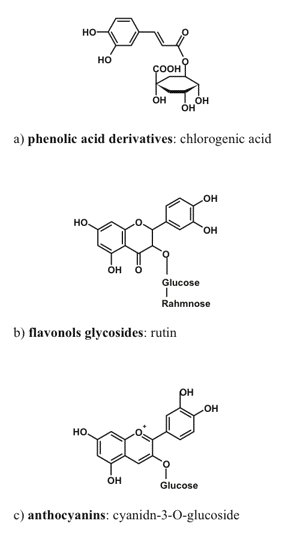
Figure 3. Typical representative of phenolic compound groups important for radiation screening in plants.
The basic structure of a phenolic compound is composed of one or more aromatic rings with hydroxyl group(s) as substituent(s). Phenolics are synthesized in chloroplasts or cytoplasm and, after glycosilation, they are transported to and accumulated within the vacuoles, or excreted into apoplast where they remain within cell wall or incorporated in the cuticle. Phenolics serve a plethora of protective functions in plants. For a long time, the main phenolic-dependent protective mechanism in plants was thought to be the defense against phytopathogens and herbivores. This paradigm has changed recently to accommodate the important photoprotective function of phenolics in plants, which is supported by a large body of experimental evidence (Close and McArthur, 2002).
The most important (in the context of radiation screening) group of phenolic compounds includes hydroxycinnamates and other phenylpropanoid derivatives (the compounds with a C6-C3 carbon backbone (Burchard et al., 2000), flavonols and anthocyanins (flavonoids possessing a C6-C3-C6 backbone; Gould, 2004; Hoch, 2001). Simple phenols and phenolic acids (C6-C1) appear to be relatively uninvolved in radiation screening, probably because of their high toxicity, thus preventing their accumulation in the amounts necessary for a screening function.
Characteristic absorption spectrum of screening-relevant phenolic compounds in the UV usually contains two bands (Figure 4). The first band peaking around 280 nm appears due to the presence of aromatic ring(s), and is detected in the spectra of all phenolics. The second, long-wave band is situated in the 300-360 nm range; the exact position of its maximum varies for different classes of phenolics. In anthocyanidins and their glycosilated forms known as anthocyanins, the maxima of the second absorption band is located in the blue-green part of the visible spectrum. For example, the long-wave absorption band of cyanidin, the predominant aglycone of anthocyanins responsible for reddish coloration of leaves and fruit in many species, is centered at 525 nm.
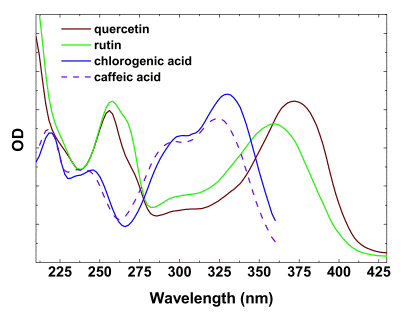
Figure 4. Absorbance spectra of certain flavonols and phenolic acids in methanol.
The molar absorption coefficients of the most of the phenolic compounds relevant to screening are within the range of 10-35 mM-1 cm-1. In solutions, flavonols and anthocyanins often undergo copigmentation. As a result, the increase of absorption coefficients, bathochromic shifts of maxima and peak flattening are observed, significantly affecting the efficiency of absorption of light by these compounds localized within the cells and tissues. In the case of the common plant flavonols (such as quercetin and kaempferol glycosides), their in planta tautomerization induces more profound bathochromic shifts of the long-wave absorption maxima, which could be particularly significant for visible radiation screening.
Betalains comprise an interesting group of water-soluble nitrogen-containing compounds of limited occurrence within flowering plants. Specifically, they are encountered mostly in the nine families of the order of Caryophyllales. Two main classes of betalains are distinguished: purple-to-rose betacyanins and yellowish betaxanthins (Figure 5).
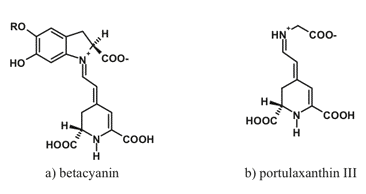
Figure 5. Typical representatives of two important betalain groups: (a) betacyanins and (b) betaxanthins.
Betalains also occur in plants as glycosides, acylglycosides or more complex species: ferulic acid esters and flavonol conjugates are synthesized as a result of UV irradiation. Absorption spectra of betacyanins are characterized by a broad band with the maximum near 593-543 nm; a bathochromic shift to 550 nm is possible as a result of intramolecular copigmentation. The spectra of betaxanthins feature three main bands with the maxima near 217, 262, and 546-471 nm. The similarity of spectral properties and subcellular localization of betalains and anthocyanins suggests that the former fulfill the function of anthocyanins in the species lacking these pigments.
Carotenoids are the accessory pigments ubiquitous in photoautotrophs. These pigments participate in light-harvesting, fulfill photoprotective function, and stabilize the pigment-protein complexes of the photosynthetic apparatus. More than 800 carotenoid species with a linear or cyclic structure have been discovered in plants thus far (Figures 6, 7).
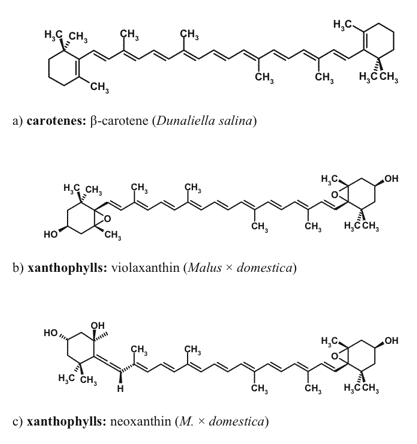
Figure 6. Carotenoids native to the photosynthetic apparatus, which can be accumulated as secondary (extrathylakoid) carotenoids.
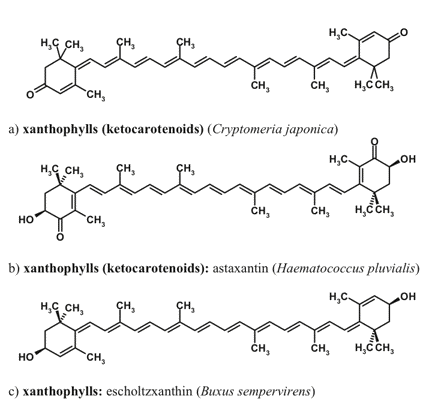
Figure 7. Carotenoids non-native to the photosynthetic apparatus, which are involved in the optical screening of visible radiation.
Carotenoids are the terpenoid compounds formed via condensation of eight isoprenoid monomers. The carotenoids of most plant species are represented by carotenes and xanthophylls, with characteristic three-headed absorption maxima in the blue part of the spectrum, 400-480 nm (Figure 8).
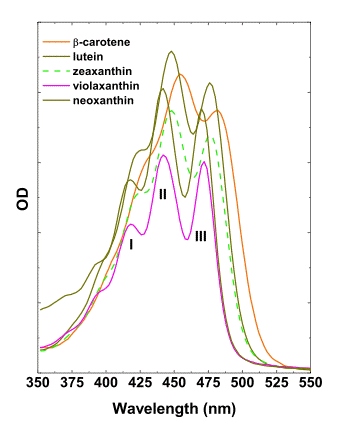
Figure 8. Absorbance spectra of most important higher plant carotenoids in acetone.
The composition of 'photosynthetic' or primary carotenoids is highly conserved, but under stressful conditions certain species accumulate unusual red-colored secondary carotenoids, such as rhodoxanthin (Merzlyak et al., 2005). The presence of conjugated keto-groups in the molecules of keto-carotenoids causes the considerable bathochromic shift of the main absorption maximum, in comparison with the carotenoids native to the photosynthetic apparatus. To the best of our knowledge, no evidence has been obtained on the involvement of rhodoxanthin or other red carotenoids in photoprotection within thylakoid membranes. Carotenoid molar absorption coefficient in the maximum located in the blue-green region of the spectrum could be as high as 180 mM-1 cm-1.
The major photosynthetic carotenoids of higher plants include ß-carotene, and a number of xanthophylls such as lutein, neoxanthin, violaxanthin, antheraxanthin, and zeaxanthin. The structures of xanthophylls of unicellular algae are much more diverse. Many microalgal species are able to accumulate secondary carotenoids that do not participate in photosynthesis and are represented by carotenoids both native (e.g., ß-carotene) and non-native to the photosynthetic apparatus (such as astaxanthin, canthaxanthin, and rhodoxanthin). The secondary xanthophylls are often accumulated in the form of fatty acid esters. Higher plants are also capable of extrathylakoid accumulation of carotenoids, mainly in the form xanthophyll fatty acid esters, whose composition is species-dependent.
Other screening pigments. There are also compounds involved in screening of solar radiation, but resembling none of the major categories. This group is growing, and will obviously continue to grow, since the vast diversity of screening compounds, especially in phototrophic microorganisms, remains to a considerable extent unexplored, and many such compounds are discovered every year. For example, lichen acids serve multiple roles in the protection of lichens from biotic and environmental stresses. In particular, lichen acids are important for the protection of the photobiont against photooxidative damage by solar radiation, which imposes considerable risks under harsh conditions characteristic of lichen. A special case is exemplified by cyanolichens, which accumulate scytonemin as screening pigments. Scytonemin is one of the most studied cyanobacterial screening compounds, often encountered in the sheath of mate-forming cyanobacteria (planktonic species mostly lack scytonemin). Scytonemin is a long-known protective compound, where participation in UV photoprotection via optical screening was rigorously confirmed.
Summary. Screening compounds greatly differ in terms of their biosynthetic origin and chemical structure, but all of them possess pronounced absorption bands with high extinction coefficients in the UV and/or visible part of the spectrum (Figures 2-8). Different taxa of photoautotrophic organisms differ in their ability to synthesize various groups of photoprotective screening pigments. Nevertheless, the combinations of screening compounds simultaneously present in the cells and tissues of many algae and plants could efficiently attenuate radiation in the very broad spectral band extending from UV to the blue-green, and even to the yellow-orange region of the visible part of the spectrum (Figure 9).

Figure 9. Absorption spectra of the representatives of key groups of photoprotective pigments, and the energy spectrum of solar radiation near the surface of the Earth. The absorption maxima of most phenolics are located in the UV-B and UV-A regions; anthocyanins possess a long-wave maximum in the green region, the shortwave part of the spectrum is not shown). This is the band where the maximum of energy in the solar spectrum is located. The photoprotective carotenoids absorb in the blue-green range. The spectra are normalized to their absorption maxima. [Reproduced from Solovchenko and Merzlyak, 2008. With kind permission from Springer Science+Business Media.]
Localization of Screening Pigments Within Plant Cells and Tissues The efficiency of photoprotection by screening pigments should strongly depend on their localization within plant tissue; it can be evaluated in terms of external filtering, when photoprotective pigments serve as a screen, or internal filtering, when such pigments compete with light absorption by chlorophyll within a leaf. A large body of evidence suggests that the bulk of high light stress-induced screening compounds are situated within the protective complex comprised by cuticle, epidermis and its appendages, and derivative structures such as hairs and trichomes (Karabourniotis and Bornman, 1999).
Screening pigments discovered in plants to the date can be divided into several categories according their subcellular localization (Figure 10).

The cellular compartment in which a pigment is or could be localized depends on the properties of its molecules (such as polarity), the site of its biosynthesis (e.g., chloroplasts or cytoplasm), and accumulation (e.g., endoplasmic reticulum or vacuole), as well as its effect on cellular metabolism. In particular, certain phenolics could be toxic to the cell when present in concentrations necessary for the efficient screening of solar radiation. These phenolics are predominantly accumulated in the form of glycosides, which are less toxic, within the vacuole where they could reach a high local concentration without the risk of damage to other cell components; the predominant vacuolar localization of phenolics in the cell is also determined by their high polarity. Thus, anthocyanins, flavonol and phenolic acid derivatives, which are accumulated in vacuoles of epidermal and underlying mesophyll or hypodermal cells, are distributed within leaf and fruit tissues according to diverse patterns (Figures 11-13).
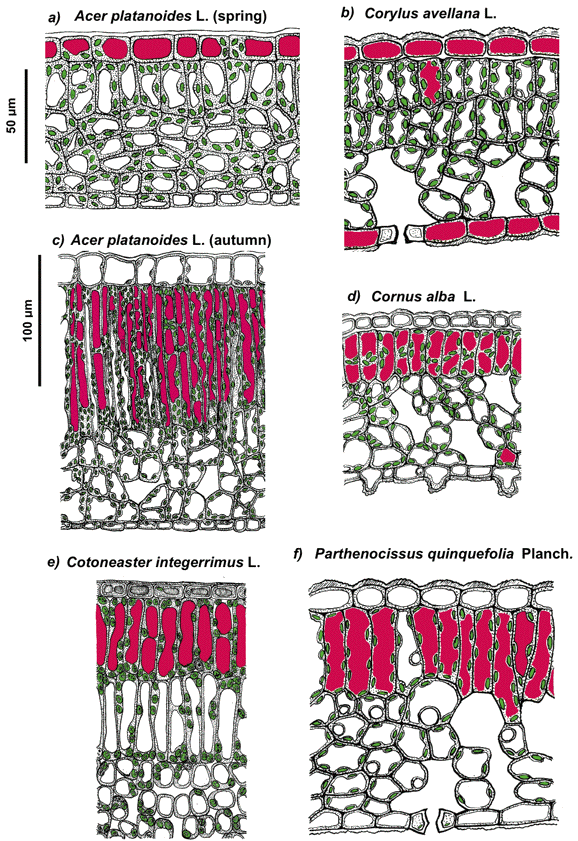
Figure 11. Schematic representation of the anatomy of spring (a, b) and autumnal (c-f) leaves of the species studied. The chloroplasts and anthocyanin-containing vacuoles are shown in green and magenta, respectively. Note that in juvenile leaves anthocyanins are accumulated in epidermal cell vacuoles whereas in autumn leaves these pigments are localizing predominantly in mesophyll. [Reproduced from Merzlyak et al. (2008). Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J. Exp. Bot. 59, 3903-3911, by permission of Oxford University Press.]
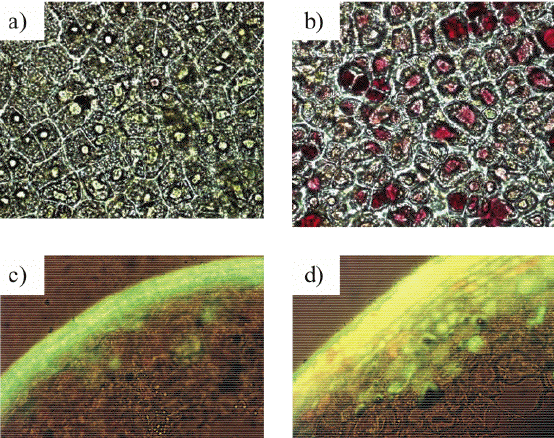
Figure 12. Microphotographs of the apple (Malus x domestica Borkh.) fruit tissues adapted to low (a, c) an high (b, d). Bright-field microscopy (x400) reveals red anthocyanins in the vacuoles of the cells of sunlit skin (b) absent in shaded tissue cells (a). Fluorescent microscopy (c, d; x200; λexcitation: 365 nm, λemission: 450-600 nm) demonstrates an increase in flavonols (apparent as bright yellow fluorescence) in the vacuoles of sunlit skin (d) over that of the shaded skin (c). Also note the presence of chlorogenic acid in the vacuoles of shaded skin cells (c) and the cuticle of the both samples (c , d).
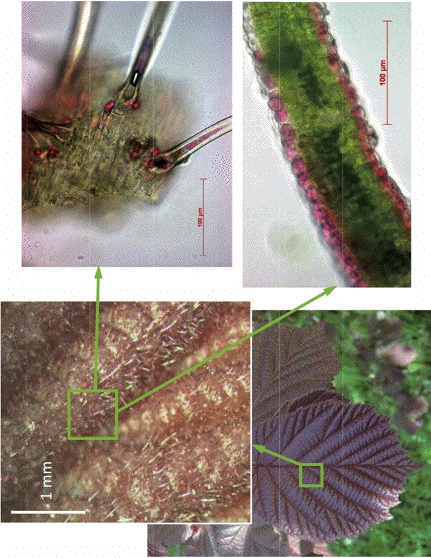
Figure 13. Juvenile leaves of walnut (C. avellana L.) and localization of anthocyanins in their epidermal (lower right plate) and hair cells (upper right plate). They appear red when observed in reflected light in spite of considerable chlorophyll content due to the presence of anthocyanin-containing hairs which strongly scatter light on their surface.
It should be noted that flavonols and other screening phenolics could be co-localized with anthocyanins. Thus, the epi- and hypodermal cells of sunlit apple skin contain a high amount of both anthocyanins and flavonols in their vacuoles. There are exceptions, however. It was found recently that certain phenolic compounds could be accumulated in relatively high amounts in chloroplasts, e.g., in the lumen. However, it is a matter of question whether these phenolics could contribute to radiation screening; it seems more likely for them to function as ROS scavengers. A considerable amount of phenolic compounds are also accumulated in the endoplasmic reticule and/or are excreted to the apoplast, where it becomes involved in lignin biosynthesis or impregnates the cell wall matrix.
Highly hydrophobic extraplastidic carotenoids, which could not be adopted by vacuole are accumulated within lipid globules forming in cytoplasm or the plastidic stroma (plastoglobuli). The formation of lipid globules comprised mostly by neutral lipids appears to be among key factors controlling the accumulation of extrathylakoid carotenoids and their fatty acid esters. Thus, the formation of cytoplasmic oil bodies, and hence its capacity for carotenoid storage, is an important factor regulating the synthesis of extrathylakoid ß-carotene in D. bardawil.
One of the most striking common features of algal cell acclimating to high-light stress is a vast increase in size and number of cytoplasmic lipid globuli (so called oil bodies), which eventually could occupy most of the cell volume. At the same time, growing oil bodies often became more electron-dense, due to an increase in unsaturation of the lipids. The lipid inclusions, occurring mainly in cytoplasm, often become the depot for lypophilic screening pigments like extrathylakoid carotenoids. Such is the case in a number of algal species such as Haematococcus pluvialis, different species of the genus Dunliella, Parietochloris incisa and a number of others. Deposition of screening carotenoids in microalgae could also take place within the chloroplast. This is the case with D. salina (D. bardawil), which accumulate high amounts of ß-carotene within the stroma of chloroplast in the form of lipid-containing granules stabilized by special proteins.
The comparison of the high light stress-induced changes in the chloroplast ultrastructure of green algae and higher plants reveals their marked similarity (Figure 14). As in microalgae, in higher plant chloroplasts, a partial degeneration of chloroplast thylakoid membranes and the formation of large in size and number of lipid globules is often observed under acclimation to high light; yet it should be emphasized that the lipid globules (oil bodies) in algal cells are characterized by extraplastidic localization, whereas lipid globules in higher plant cells (plastoglobuli) are localized within chloroplasts.
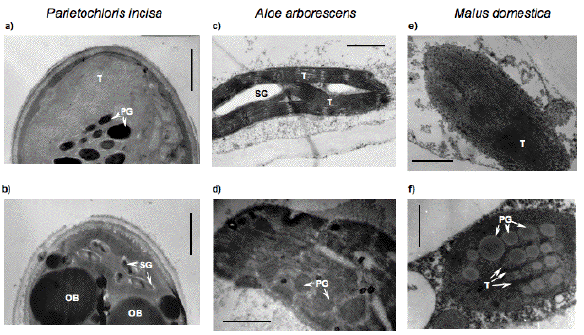
Figure 14. The changes in plastid ultrastructure of microalgae (a, b) and higher plant leaves (c, d) and fruit (e, f) during their adaptation to high fluxes of light. As a rule, these processes are accompanied by degeneration of the granae and lamellae of the chloroplasts, increase in size and number of plastoglobuli and cytoplasmic oil bodies (b, d, f). SG = starch grains; OB = oil bodies; P = plastoglobuli; T = thylakoids. The scale bar corresponds to 1 or 0.5 µm for the panels a, c, e or b, d, f, respectively.
Assessment of Screening Pigment Efficiency in situ
The optical properties of screening pigments in planta differ considerably from that of isolated pigments. As a result, the effective screening ability of the pigments within plant cells and tissues could be considerably higher than one can expect from studying their spectra in solution. In particular, broadening of absorption bands and bathochromic shifts considerably expands the spectral region where screening pigments could potentially provide photoprotection in vivo. These circumstances emphasize the importance of investigations on the in planta spectroscopy of screening pigments to gain an insight into their real photoprotective efficiency.
The efficiency of photoprotection provided by screening pigments in planta is determined by the ratio of the amount of radiation intercepted by screening pigments, and the amount of radiation absorbed by photosynthetic pigments and other photosensitizers present in plant cell. Numerous approaches characterized by distinct advantages and drawbacks are currently employed for the quantification of screening pigments, and the estimation of their efficiency. A common approach involves spectrophotometric analysis, and comparison of the absorption spectra of the extracts of algal cells or higher plant tissues grown under contrasting conditions (i.e., normal and stressful conditions promoting the accumulation of screening pigments).
A number of works dedicated to the investigation of the spectral absorption of light by superficial structures and epidermal tissues of leaves and fruit was carried out on the preparations of isolated cuticle and epidermis. This method does provide information on the attenuation of light by plant superficial structures, but suffers from uncertainties related to the isolation and nativity of the preparation. Therefore, experiments with optical microfibers introduced to the mesophyll of otherwise intact leaf are of a considerable interest; the findings obtained using this technique allowed the description of the light gradients within the leaf blade for different plant species.
Recently, non-destructive techniques were developed based on the analysis of optical reflectance or fluorescence excitation spectra of chlorophyll employing the latter as the endogenous fluorescent probe (Agati et al., 2002; Bilger et al., 2001). The ratio of the intensities of chlorophyll fluorescence excited in the UV-B to that in the blue-green regions of the spectrum was found to be proportional to the UV-B transmittance of the epidermis samples, and their phenolic content. In leaves, the ratio of chlorophyll fluorescence excited by UV-B to that excited by blue-green light showed a negative correlation with the concentration of whole-leaf UV-B-absorbing pigments, and a positive correlation with the transmittance of isolated epidermal tissue, where flavonoids accumulate. In these studies, screening by flavonoids was quantified, on the leaf/fruit level, by using a chlorophyll fluorescence excitation (CFE) ratio, e.g., the ratio of the chlorophyll fluorescence yields for different excitation wavelengths. Further progress can be made only by relating a CFE spectrum to specific spectral features of chlorophyll and individual light-screening and/or internally-trapping pigments in the specimen under examination.
Accumulation of MAA and Scytomenin Increases the UV-resistance of Photoautotrophs
The UV-protective role of mycosporin-like amino acids (MAA) and scytonemin in prokaryotic photoautotrophs and eukaryotic microalgae is relatively well established (for reviews, see (Cockell and Knowland, 1999; Shick and Dunlap, 2002). These studies have documented MAA concentration-dependent protection of growth and photosynthesis in algae. In particular, MAA alleviated the irradiation-induced chlorophyll photobleaching and photosynthesis inhibition in desiccated cyanobacteria. Also, higher concentration of MAA (and other UV-absorbing materials) in the hosts' cells protect the photosynthesis of symbiotic microalgae in hospite, whereas UV does inhibit photosynthesis in the freshly isolated endosymbionts (Shick and Dunlap, 2002), and references therein).
One of the most harmful effects of UV irradiation is the damage to DNA, resulting in abnormal gene expression or mutations through incorrect DNA replication or repair. There is an inverse correlation between UV screening pigment content and (UV-B)-induced DNA damage in several species of red marine algae. The importance of MAA for the protection of nucleic acids was confirmed with measurements of the fluorescence of DAPI-labeled cells (Shick and Dunlap, 2002).
Scytonemin-synthesizing cultures were more resistant to photoinhibition of photosynthesis by UV-A than cultures lacking scytonemin. The presence of this screening compound was correlated with the inability of UV-A radiation to induce strong photosynthetic pigment fluorescence (685 nm emission), regardless of the specific content of the photosynthetic pigments (Garcia-Pichel et al., 1992). Importantly, the protective function of this compound is more evident under conditions imposing physiological inactivity such as desiccation, when 'active' photoprotective mechanisms are less efficient. The physical removal of the scytonemin-containing extracellular envelopes brought about the loss of UV-A resistance.
Anthocyanins and Other Phenolics as a Shield Against UV and Excessive PAR
An important role in the UV-protection of plants is played by UV-absorbing phenolics. Thus, plants grown in glasshouses blocking most of the solar UV contain less flavonols and phenolic acids in comparison with plants of the same species grown outdoors. Accordingly, the former plants displayed an increased UV susceptibility (Mazza et al., 2000). Investigations of the mutants deficient in the synthesis of flavonoids with different spectral characteristics and localizations as well as transgenic plants also confirmed the importance of the phenolics in UV defense. Further evidence of tight relationships between UV-resistance and the ability to synthesize certain screening phenolics were provided by inhibitory analysis with and the phenylalanine ammonia-lyase (PAL) inhibitor, 2-amino-indan-2-phosphonic acid (AIP). According to Gitz at al. (1998), plants grown with 50 µM AIP were about twice as sensitive as a control plant to UV-B damage of photosystem II, suggesting that phenylpropanoids carried over from the seed, as well as flavonoids, serve as UV screens in young red cabbage seedlings.
The photosynthetic apparatus of higher plants is particularly sensitive to damage by UV-B radiation. The primary targets of UV-B radiation in photosystem II are the 32 kDa D1 protein of the reaction center, and the water-oxidizing system. The extent of the (UV-B)-induced decrease in the maximum chlorophyll fluorescence level (Fm), and potential quantum yield of photosystem II, as measured via variable chlorophyll fluorescence (Fv/Fm), exhibited a high correlation with apple skin flavonol content (Solovchenko and Schmitz-Eiberger, 2003; see also Figure 15). The phenomenon of (UV-B)-dependent inhibition of maximum photochemical yield of photosystem II inversely correlated with the build-up of epidermal screening for UV-B radiation, and was recorded in the leaves of a number of plant species. A marked reduction in the efficiency of photosystem II has been recorded after exposing artificially de-haired leaves (screening phenolics are localized predominantly in the hairs) to UV-B radiation.
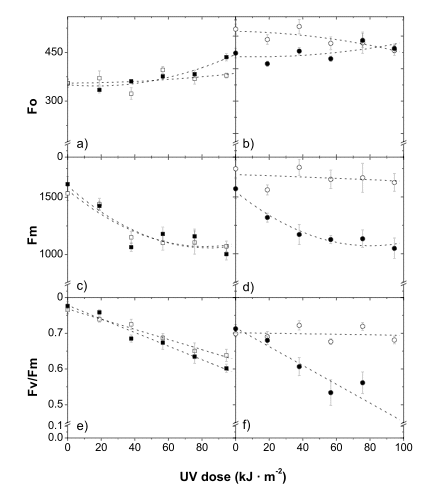
Figure 15. Changes in chlorophyll fluorescence in shaded (closed symbols) and sun-exposed (open symbols) skin in the course of UV-B irradiation of Granny Smith (a, c, e) and Braeburn (b, d, f) apples. In Granny Smith apples, which are susceptible to (UV-B)-damage, a strong solar light-induced increase in flavonol content is much less pronounced, in comparison with Braeburn apples, which are highly resistant to UV-B radiation. [Reproduced from Solovchenko and Schmitz-Eiberger, J. Exp. Bot., 2003, 54 (389), 1981, by permission of Oxford University Press.]Anthocyanins appear to be one of the most investigated groups of 'stress-pigments' (Chalker-Scott, 1999). The induction of their synthesis represents a well-known, obvious and common high light-induced response. The physiological significance of anthocyanins remain a subject of vigorous debate. There are numerous experimentally confirmed examples of an increase in the resistance of plant photoassimilatory tissues to photodamages as a result of anthocyanin accumulation. Thus, anthocyainin-containing dogwood (Cornus sericea L.) leaves were less susceptible to photoinhibition, and displayed a higher efficiency of photosystem II in comparison with acyanic leaves of the same species.
A considerable problem in studies of the physiological significance of anthocyanin accumulation under stress is imposed by the difficulty in finding samples differing in anthocyanin content, but otherwise similar. This obstacle could be circumvented by selecting the samples with similar absorption in red (rather than similar chlorophyll content), and different absorption in the green region governed by anthocyanins. For example, Smillie and Hetherington (1999) used white, red and blue-green light to subject pods of red (anthocyanin-containing) and green (anthocyanin-free) Bauhinia variegata L. phenotypes to photoinhibitory conditions.
Red light, which is not absorbed by anthocyanins, induced a similar degree of photoinhibition in pods of both colors. The increased tolerance of red pods for blue-green and white light irradiation compared with green pods was attributed to the presence of anthocyanins. This was, according to Steyn et al. (2002), first conclusive evidence supporting a photoprotective function for anthocyanins that was not obviously confounded by other photoprotective measures. A similar approach was used by Field et al. (2001) to demonstrate that anthocyanins reduced photodamage in red compared with yellow senescing leaves of red-osier dogwood, and Merzlyak et al. (2008) studied the efficiency of radiation screening by anthocyanins.
It should be noted though that photoprotection by anthocyanins could come at a cost: the absorbance of light by anthocyanin in the visible region of the spectrum causes some reduction in net carbon gain under limiting light, whereas under saturating light this effect could turn to be negligible. Mesophyll cells located below a light-filter comprised by anthocyanin-containing epidermal cells assumed the characteristic photosynthetic features of shade-type cells. As a result, red leaves showed a 23% reduction in CO2 assimilation under light-saturating conditions, and a lower threshold irradiance for light-saturation, relative to those of green leaves. Limitation of light penetration by anthocyanins could, in certain cases, limit the efficiency of the photorepair of cyclobutane pyrimidine dimers (CPD) formed as a result of UV-B irradiation (Hada et al., 2003).
Carotenoid Screening Pigments Protect from Photodamage
Unlike that of anthocyanins and UV-absorbing phenolics, the physiological significance of carotenoids as screening pigments is much less studied. Nevertheless, there are reports on the participation of extrathylakoid carotenoids in the screening of excessive radiation in the blue-green part of the visible spectrum. The screening by secondary carotenoids was initially documented under stressful conditions in carotenogenic microalgae (Bidigare et al., 1993; Hagen et al., 1994). The secondary carotenoids rendered the algal cells less susceptible to photodamage by elevated PAR and UV fluxes as well as to exogenous photosensitizers. Thus, the cells of the chlorophyte H. pluvialis, featuring high contents of astaxanthin esters, retained a high efficiency of photosystem II even under high PAR irradiance, causing photoinhibition in green astaxanthin-free cells. The formation and deposition of astaxanthin seems to prevent a profound reduction in D1 protein level, enabling the cell to maintain photosystem II function and structural integrity. Interestingly, in the course of recovery of the cells from high-light stress, the astaxanthin globules concentrated around the nucleus, indicating that the pigment also serves as a physicochemical barrier, protecting the replicating DNA from oxidative damage during cell division.
Ben-Amotz et al. (1989) demonstrated that the massively accumulated ß-carotene, e.g., in Dunaliella bardawil, protects against photoinhibition by visible radiation in the bands strongly absorbed by ß-carotene (i.e., in the blue). No photoprotection is observed during irradiation with red light, which is not absorbed by ß-carotene. This is in agreement with the observation on the location of the ß-carotene globules, distant from the thylakoid-bound chlorophyll, and with the mechanism of the photoprotection by massively accumulated carotene is a screen.
In higher plants, the radiation screening function of secondary carotenoids appears to be even less studied than in microalgae. The evidence of the existence of carotenoid-based screening in higher plants appeared only recently (Ida et al., 1995; Han et al., 2003: Hormaetxe et al., 2005).
Concluding Remarks
Numerous lines of evidence point to the great physiological significance of screening pigments in microalgae and higher plants. There were earlier indications of the possible involvement of numerous compounds in radiation screening, but solid evidence began to come in during last 15 years. More importantly, in many cases quantitative relationships between the amount and/or spectral properties of accumulated screening pigments, and an increase in the resistance to photodamage, have been established. Taking into account prominent achievements in the research on UV-screening compounds, the existence and operation of the screening-based mechanisms in all major taxa of photoautotrophs including cyanobacteria and plants now seems to be established.
The screening in the visible part of the spectrum, especially in the instance of secondary carotenoids, is much less certain and a more controversial issue. Nevertheless, there is a considerable progress in unraveling the role of visible radiation screening compounds in protecting plants against photodamages, this is especially true for anthocyanin pigments, which draw the increasing attention of researchers. To conclude, one could think of radiation screening by extrathylakoid pigments as a photoprotective mechanism relying on principles totally different from that of 'classic' photoprotective mechanisms, but integral to the whole system of protection of plants against photooxidative stress. Screening-based photoprotection is a first-line of defense of plants against potentially harmful solar radiation, which takes a considerable time to deploy as well as to withdraw, and is therefore effective for the long-term photoacclimation of plants.
References
Agati, G., Galardi C., Gravano, E., Romani, A., and Tattini M. (2002). Flavonoid distribution in tissues of Phillyrea latifolia L. leaves as estimated by microspectrofluorometry and multispectral fluorescence microimaging. Photochemistry and Photobiology 76(3): 350-360.
Asada, K. (2006). Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology: 391-396.
Ben-Amotz, A., Shaish, A. , and Avron M. (1989). Mode of action of the massively accumulated ß-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiology 86: 1286-1291.
Bidigare, R., Ondrusek, M. , Kennicutt, M.C., Iturriaga, R., Harvey, H.R., Hoham, R.W., and Macko, S.A. (1993). Evidence a photoprotective for secondary carotenoids of snow algae. Journal of Phycology 29(4): 427-434.
Bilger, W., Johnsen, T., and Schreiber, U. (2001). UV-excited chlorophyll fluorescence as a tool for the assessment of UV-protection by the epidermis of plants. Journal of Experimental Botany 52(363): 2007-2014.
Burchard, P., Bilger, W., and Weissenbck, G. (2000). Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV-A and UV-B radiation in developing rye primary leaves as assessed by ultraviolet-induced chlorophyll fluorescence measurements. Plant, Cell & Environment 23(12): 1373-1380.
Chalker-Scott, L. (1999). Environmental significance of anthocyanins in plant stress responses. Photochemistry and Photobiology 70(1): 1-9.
Close, D. and McArthur C. (2002). Rethinking the role of many plant phenolics-protection from photodamage not herbivores? Oikos 99(1): 166.
Cockell, C. and Knowland J. (1999). Ultraviolet radiation screening compounds. Biological Reviews 74(03): 311-345.
Demmig-Adams, B. and Adams, W. (2006). Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytologist 172(1): 11-21.
Garcia-Pichel, F., Sherry, N., and Castenholz R.W. (1992). Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chiorogloeopsis sp. Photochemistry and Photobiology 56(1): 17-23.
Gitz, D., Liu, L., and McClure, J. (1998). Phenolic metabolism, growth, and UV-B tolerance in phenylalanine ammonia-lyase-inhibited red cabbage seedlings. Phytochemistry 49(2): 377-386.
Gould, K. (2004). Nature's Swiss army knife: the diverse protective roles of anthocyanins in leaves. Journal of Biomedicine and Biotechnology 5(2004): 314-320.
Hada, H., Hidema, J., Maekawa, M., and Kumagai, T. (2003). Higher amounts of anthocyanins and UV-absorbing compounds effectively lowered CPD photorepair in purple rice (Oryza sativa L.). Plant, Cell & Environment 26: 1691-1701.
Hagen, C., Braune W. , and Bjrn, L. (1994). Functional aspects of secondary carotenoids in Haematococcus lacustris (Volvocales). III. Action as a sunshade. Journal of Phycology 30(2): 241-248.
Han, Q., Shinohara, K., Kakubari, Y., and Mukai, Y. (2003). Photoprotective role of rhodoxanthin during cold acclimation in Cryptomeria japonica. Plant, Cell & Environment 26(5): 715-723.
Hoch, W., Zeldin E., and McCown, B. (2001). Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiology 21(1): 1.
Hormaetxe, K., Becerril, J., Fleck, I., Pint¢, M., and Garcia-Plazaola, Jet. (2005). Functional role of red (retro)-carotenoids as passive light filters in the leaves of Buxus sempervirens L.: increased protection of photosynthetic tissues? Journal of Experimental Botany 56(420): 2629-2636.
Ida, K., Masamoto, K., Maoka, T., Fujiwara, Y., Takeda, S., and Hasegawa, E. (1995). The leaves of the common box, Buxus sempervirens (Buxaceae), become red as the level of a red carotenoid, anhydroeschscholtzxanthin, increases. Journal of Plant Research 108(3): 369-376.
Field, T., Lee, D., and Holbrook, N. (2001). Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiology 127: 566-574.
Karabourniotis, G. and Bornman J. (1999). Penetration of UV-A, UV-B and blue light through the leaf trichome layers of two xeromorphic plants, olive and oak, measured by optical fibre microprobes. Plant Physiology 105(4): 655-661.
Mazza, C., Boccalandro, H., Giordano, C., Battista, D., Scopel, A., and Ballare, C. (2000). Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiology 122(1): 117-126.
Merzlyak, M., Solovchenko, A., and Pogosyan, S. (2005). Optical properties of rhodoxanthin accumulated in Aloe arborescens Mill. leaves under high-light stress with special reference to its photoprotective function. Photochemical and Photobiological Sciences 4(4): 333-340.
Merzlyak, M. N., Melo, T. B., and Naqvi K.R. (2008). Effect of anthocyanins, carotenoids, and flavonols on chlorophyll fluorescence excitation spectra in apple fruit: signature analysis, assessment, modelling, and relevance to photoprotection. Journal of Experimental Botany 59(2): 349-359.
Shick, J. and Dunlap, W. (2002). Mycosporine-like amino acids and related gadusols: biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annual Review of Physiology 64(1): 223-262.
Sinha, R. and Hder, D. (2007). UV-protectants in cyanobacteria. Plant Science 174: 278-289.
Smillie, R. and Hetherington, S. (1999). Photoabatement by anthocyanin shields photosynthetic systems from light stress. Photosynthetica 36(3): 451-463.
Solovchenko, A. and Merzlyak, M. (2008). Screening of visible and UV radiation as a photoprotective mechanism in plants. Russian Journal of Plant Physiology 55(6): 719-737.
Steyn, W., Wand, S., Holcroft, D., and Jacobs, G. (2002). Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytologist 155(3): 349-361.
12/19/10