Melanopsin: A Photopigment Regulating Circadian Photoentrainment May Lead to a Blue Light-Induced Treatment of Diabetes
Anamika Sengupta
Center for Teaching and Learning
Ross University School of Medicine
Commonwealth of Dominica, West Indies
asengupta@rossmed.edu.dm
Introduction
Over the past decade there is growing acceptance that photoreception in the retina is not merely restricted to the rods and cones, the classical photoreceptors. There is mounting evidence that a small population (0.2%) of retinal ganglion cells (RGCs), designated as intrinsically photosensitive retinal ganglion cells (ipRGCs), are capable of responding to light [1, 2]. The presence of melanopsin, a blue light sensitive pigment in the ipRGCs [1, 3, 4], accounts for their direct photosensitivity. With the largest melanopsin positive dendritic tree diameter [5], ipRGCs serve as primary conduits through which photic information is relayed from the retina to non-image forming visual centers of the brain, namely the olivary pretectal nucleus (OPN), and the suprachiasmatic nucleus of the hypothalamus (SCN). As a photo sensory pigment, melanopsin is involved primarily in photoentrainment or alignment of the biological clock of an organism with the environmental dusk and dawn cycle. In contrast to the classical photopigment rhodopsin (committed to image-forming visual functions), melanopsin is associated with mediation of non-image forming visual functions, including pupillary light reflex and circadian entrainment [6, 7, 8, 9]. Melanopsin requires light stimulus of a higher irradiance and longer duration for activation [10], while rhodopsin is optimized for rapid and sensitive contrast acuity. In recent years, identification of melanopsin cell projections in the dorsal lateral geniculate nucleus (LGN) is indicative of its involvement in visual perception as well [11, 12]. The existence of a cross-talk between dopamine and melanopsin in the context of light responsiveness of ipRGCs [13] is eminently interesting. The association of the melanopsin system in allodynia (pain) to light or photophobia [14], sleep [15], mood regulation [16], and the recent popularity of melanopsin as an optogenetic tool [17, 18] further emphasizes the clinical importance of this retinal photosensory pigment. This modest mini review aims at summarizing the importance of melanopsin as a photosensory pigment, the genesis of which begins with its in vivo function as a circadian photoentrainer in mammals, to its possible future role as molecular switch for light induced regulation of intracellular synthetic transcription/translation machineries in therapeutic cell implants.
The Blue Light Sensitive Pigment Melanopsin
Originally cloned from Xenopus dermal melanophores [19], the melanopsin gene (Opn4) has been described in a variety of vertebrates [20, 21, 22,]. Although the gene has various orthologs [23], Opn4m is considered to be the mammalian ortholog [24, 25]. In humans, the melanopsin gene, expressing mainly in the ipRGCs, produces mature melanopsin protein, which is an opsin subgroup of G protein (guanosine nucleotide binding protein) coupled receptors [26] linked to a chromophore containing 11-cis retinal (specific form of vitamin A). The membrane density of melanopsin is 104 fold lower in ipRGCs when compared to that of rhodopsin in rod cells [27]. Its peak spectral sensitivity is around 480 nm [27, 28], thus it is highly sensitive to blue light. Characterized by low photon catch, melanopsin also exhibits a low phototransducing ability in bright light [27]. The light transduction mechanism of melanopsin is different from the classical photosensory pigments. Irradiation of rod/cone opsins by bright light decreases cGMP levels closing cGMP-gated membrane channels leading to membrane hyperpolarization and activation of the photoreceptors [29, 30]. Conformational changes of melanopsin, due to photoisomerization of
11-cis retinal by blue light, activates phospholipase C (PLC), and phosphokinase C (PKC) triggers calcium influx into the ipRGCs. This sequence of events leads to membrane depolarization [31, 32] and subsequent activation of ipRGCs. The distinct functional roles associated with the classical photosensory pigments (present in rods/cones) and the blue light sensory pigment melanopsin are illustrated in Figure 1.
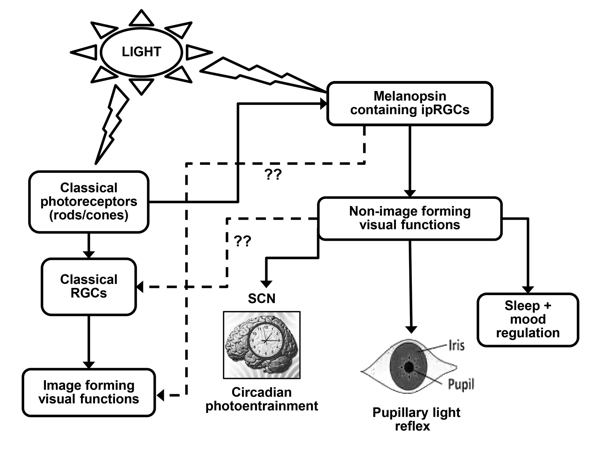
Figure 1: Regulation of Image and Non-image Forming Visual Functions of the Eye. The melanopsin positive ipRGCs respond directly to ambient light and mediate a variety of non-image forming visual functions, namely circadian photoentrainment of the SCN, pupillary light response and regulation of sleep and mood. The visual functions of the eye are regulated by the classical photosensory pigments present in rods and cones through the population of classical/non-melanopsin RGCs. The role of classical RGCs in non-image forming visual functions, and the role of melanopsin in image forming, have been suggested, but the underlying mechanism still remains unknown (??). RGC (retinal ganglion cells); ipRGCs (intrinsically photosensitive retinal ganglion cells); SCN (suprachiasmatic nucleus).
Classical Functions of Melanopsin
Circadian Photoentrainment: The circadian rhythms of our physiology and behavior are regulated by the hypothalamic SCN, the master circadian oscillator, which requires regular synchronization with the daily and seasonal fluctuations of the environmental photoperiod. This process is defined as circadian photoentrainment. Melanopsin serves as a primary candidate (among other retinal photoreceptors) regulating circadian photoentrainment of the SCN [33]. Along with pituitary adenynyl cyclase activating peptide (PACAP), which co-expresses in the ipRGCs [34, 35], melanopsin forms the retinohypothalamic tract [6, 36] that projects into the SCN and synchronizes it with the solar day [37]. The severely attenuated phase resetting response exhibited by melanopsin null mice (Opn4-/-) in response to brief pulses of monochromatic light [33], provides support for this fact. The role of classical photosensory pigments, namely the medium wavelength opsin (MW cones) in the process of photoentrainment, cannot be ignored [38, 39]. The influence of melanopsin is not only restricted to the master circadian oscillator. Melanopsin mediated regulation of autonomous organ specific molecular clocks, specifically the retinal circadian clock [40, 41, 42], has recently been established [43]. Melanopsin through dopamine is reported to regulate clock gene expression (period 1 and 2 genes) in the retina [43]. Studies contradicting the role of melanopsin in circadian photoentrainment through direct photic input to the SCN also exists [44], but at this particular point they are far outweighed by the merits of those studies that have successfully established the fact.
Pupillary Light Response: In mammals, melanopsin expressing ipRGCs project into the LGN and olivary pretectal nucleus (OPN), which are the centers controlling pupillary light reflex [45, 5]. Existing literature speaks of the involvement of ipRGCs in the regulation of baseline pupil diameter [46], the steady state pupil diameter [47], and post-illumination pupillary response [48]. The existence of a functional melanopsin driven inner retinal pathway controlling post-stimulus sustained pupillary response, has been identified in humans [49, 46, 50], as well as in other mammals like mice and macaque monkeys [48, 51]. The fact that melanopsin is required for full pupillary constriction at high irradiance, while the contribution of classical photoreceptors (rods and cones) to pupillary control mechanisms is restricted to low irradiance, has been established [52]. The expression of melanopsin in the iris muscles of the eye [53] is a notable finding that lends additional support to the role of melanopsin in the regulation of the pupillary light response.
Pupillary responses differ as a function of light intensity and wavelength, reflecting phototransduction primarily mediated by rods, cones, or melanopsin [54]. Based on this fact, the use of chromatic light stimuli to elicit transient and sustained pupillary light reflexes may well translate into a clinical pupillary test in the near future, allowing differentiation between disorders affecting photoreceptors and those affecting retinal ganglion cells [55, 56]. Melanopsin regulated post-illumination pupil response (PIPR) may be important for documenting inner retinal function in patients with diabetes without diabetic retinopathy [57].
Regulation of Sleep and Mood: The daily cycle of sleep and wakefulness in humans is regulated by brain circuitry and neurotransmitters [58]. The hypothalamus, and more specifically the SCN, is the switch that shuts off the arousal system during sleep [58, 59, 60]. Melanopsin conveying non-visual light information to the SCN plays a crucial role in mediating the effects of light on sleep [15]. Loss of sleep homeostasis in vertebrates induced by a lack of melanopsin expression [15] supports the direct photic regulation of sleep by melanopsin based phototransduction [61, 62].
Current studies are addressing the role of melanopsin in the pathogenesis of circadian and sleep abnormalities associated with neurodegenerative disorders [63, 64]. Recurrent depressions in fall and winter are common in patients with seasonal affective disorder (SAD), as the hormones regulating mood and sleep become unbalanced due to seasonal changes. Individuals with low levels of melanopsin are found to frequently suffer with mood and sleep disorder [65]. Melanopsin gene variants (a single missense variant, P10L of Opn4 gene) are associated with increased risk of SAD in humans [66]. The seasonality of these depressive episodes in SAD patients and the favorable anti-depressive behaviors in response to light therapy (particularly blue light in the range of 470 nm) is indicative of the fact that melanopsin sensitivity has a unique effect of resetting the body’s internal clock and thus restoring the sleep-wake cycle and mood issues in SAD patients [67, 68].
Optogenetics and the Importance of Melanopsin as an Optogenetic Tool
Optogenetics is an emerging research discipline that utilizes the capability of light to process biological information in a fast and precise manner. Genetic targeting and expression of light sensitive functional components as optogenetic tools in target cells have enabled researchers to precisely regulate specific physiological processes in these cells in a light responsive manner. Channelrhodopsins, rhodopsin [69], halorhodopsins, and melanopsin are some commonly used opsin-based, single-component optogenetic tools in mammalian cells [70, 71]. In recent years, optogenetics has achieved impressive progress in neuroscience, enabling electrical activity in the neurons to be driven or silenced by pulses of light [72]. Scientists have successfully fused light sensitive switches to important enzymes of cellular transcription/translation machineries [73]. With the introduction of synthetic biology, complex genetic networks in mammalian cells can now be optimized to respond to physical/chemical/biological signals in a predictable manner. Synergistic partnership between optogenetics and synthetic biology has further enabled modern researchers to engineer light sensitive synthetic signaling cascades to control those networks in the target cells [74, 75]. These engineered synthetic regulatory cascades may have diverse applications in medical science, ranging from diagnostics to therapeutics in the near future [76].
The use of melanopsin as an optogenetic tool, or molecular switch, is favored over other photosensitive pigments (channelrhodopsin or
optoα1-AR) due to its high sensitivity to blue light, long lasting activation of intracellular signaling cascades, and influence on intracellular calcium dynamics [71]. Melanopsin most commonly uses a multistep intracellular G(q/11)-coupled signaling cascade [77], with significant signal amplification at each step of the cascade [14]. Constitutive expression of melanopsin in any mammalian cell allows blue light (450 nm) triggered transcription control of the PNFAT reporter through the following steps. Blue light-induced photoisomerization of the melanopsin-bound chromophore 11-cis-retinal results in a conformational change in melanopsin. This is followed by sequential activation of the Gαq-type G-protein, phospholipase C (PLC), phosphokinase C (PKC) and an influx of Ca+2 into the cytosol (potentially by release of Ca+2 from the endoplasmic reticulum) by activation of transient receptor potential channels (TRPCs). The calcium influx activates the calcium sensor calmodulin (CaM) linked to the transcription factor NFAT (nuclear factor activated T cells). CaM activates the serine/threonine phosphatase calcineurin (CaN), which dephosphorylates the serine-rich region in the N-terminus of the NFAT. This leads to the exposure of a nuclear import signal and translocation of NFAT from the cytoplasm into the nucleus. Within the nucleus, NFAT binds to its cognate promoter (PNFAT), and induces expression of a transgene in the target cells [17, 18].
Successful Regulation of Blood Glucose Homeostasis by Melanopsin Mediated Blue Llight Therapy in Mammals
In a novel study by Ye et al. [17], and described later by Auslander and Fussenegger [78], attempts were made to rewire melanopsin induced intracellular Ca+2 surge with Ca+2 CaM dependent activation of CaN and mobilization NFAT in vitro. Rodent and human embryonic kidney cell lines were cotransfected with the constitutive melanopsin expressing vector pHY42 [pHY42: PhCMV-melanopsin-pASV40 vector is composed of human melanopsin with a human cytomegalovirus (CMV) promoter, a bovine growth hormone polyadenylation (polyA) signal, and the gene that is resistant to the antibiotic neomycin], and PNFAT driven luciferase reporter construct (PNFAT-luc2P-pASV40; this vector contained an NFAT response element followed by luciferase, a poly A signal, and an antibiotic resistant gene). Activation of luciferase reporter construct by a 24 hr exposure to pulses of blue light exhibited the highest availability of intracellular melanopsin compatible G proteins in human embryonic kidney cell lines (HEK-293 cells). The efficiency of the intracellular NFAT signaling pathway was high, with no detrimental effects on cell survival (as expected due to a long term exposure of cells to blue light). Transgene expression in the HEK-293 cells could be effectively blocked by administration of calcium channel blockers in a dose dependent manner, providing further validation of the experiment [17].
In the next step, validation of this light triggered transcription control in a therapeutic setting was attempted. Keeping in mind the well-established potency of glucagon-1 like peptide (GLP-1) for treating type 2 diabetes [79], due to its glucose dependent insulinotropic actions [80, 81, 82], PNFAT induced expression of GLP-1 in HEK-293 cells engineered for constitutive melanopsin production was attempted. GLP-1 synthesized and secreted in the culture supernatant by a blue light activated synthetic transcription device in the HEK 293 cells, was found to be sufficient to induce insulin secretion in a beta cell line [78]. To further test the potential of this optogenic synthetic transcription device under in vivo conditions, pHY42/pHY57-transgenic HEK 293 cells (capable of expressing GLP-1 peptide in response to blue light) were microencapsulated in alginate-poly-(L)-lysine-alginate capsules, and were subcutaneously implanted into wild-type mice as well as diabetic mice (mice induced with human type 2 diabetes). The mice were exposed to blue light for 48 hours [17, 78]. Serum levels of GLP-1 and insulin were monitored. A significant elevation of serum GLP-1 and insulin levels were noted in the wild-type mice exposed to blue light, when compared to their non-illuminated control group (Figure 2). After a meal, glucose homeostasis remarkably improved in the diabetic mice, due to higher serum GLP-1 and insulin levels. Based on the success of this study and the long proven potential of GLP-1 therapy to improve glucose homeostasis in diabetic patients [79, 83], it was claimed that the light triggered expression of GLP-1 might evolve as a prospective treatment of glucose related pathologies in the near future [17, 76]
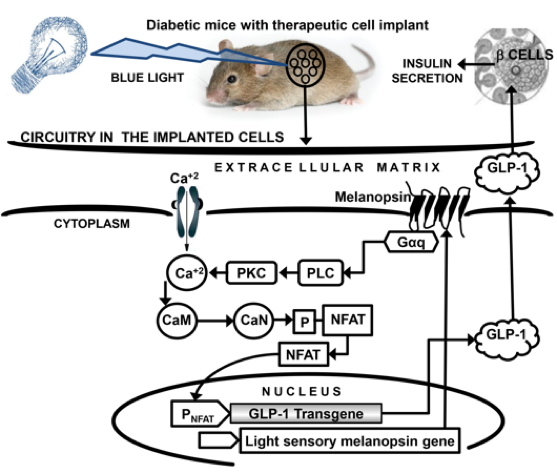
Figure 2: Phototransduction Cascade Activated by Melanopsin in Response to Blue Light in Therapeutic Cell Implants. Melanopsin-mediated release of GLP-1 and insulin in diabetic mice (mice induced with human type 2 diabetes) bearing engineered transgenic therapeutic cell implants in response to blue light. Gαq (Gαq-type G protein); PLC (phospholipase C); PKC (phosphokinase C); CaM (calmodulin); CaN (calcineurin); NFAT (nuclear factor activated T cells); PNFAT (NFAT promoter), GLP-1 (glucagon-1 like peptide)
The Prospect of GLP-1 Therapy: A Future Alternative to Existing Insulin Therapy
The success of the above mentioned studies [17, 78] led these scientists to envision that light induced GLP-1 therapy may transition from bench side to bedside in the days to come. If it does, it might be a popular replacement to the existing insulin therapy as treatment for diabetes that requires insulin. A diabetic patient could get a subcutaneous cell implant (containing blue-light sensitive melanopsin and GLP-1 expressing transgenic cells), encapsulated in a semi-permeable device as an outpatient in a doctor’s office [84].The semipermeable device would provide protection against the host immune system [85]. The implant would be attached to a light emitting diode (LED) lamp (as a source of blue light) and protected from exposure to environmental light. When required (after meals) the patient could switch on the LED lamp by pushing a button, exposing the subcutaneous implant to blue light for a certain time period. The melanopsin mediated secretion of GLP-1 from the implanted cells into the patient’s bloodstream would effectively regulate the after-meal glucose homeostasis through induction of insulin secretion from the pancreatic beta cells of the patient. After a certain period of time, when serum GLP-1 levels were sufficiently high, the LED lamp could then be turned off.
Though evidently close to success, clinical licensing of such therapeutic cell implants would only be possible after careful consideration of ethical, scientific and safety issues [78, 86]. The common questions that would need to be addressed for this purpose might be: which cell lines would be sufficiently safe for implantation into humans? How long could such an implant remain functional? Could implanted transgenic cells cross talk with the normal host tissue? If yes, what would be the consequences?
Success of Melanopsin as a Molecular Switch in the Regulation of Differential Cell Functions
The use of melanopsin as a molecular switch for the regulation of a wide array of functions in a wide variety of cell types is currently under way with much success. Melanopsin mediated regulation of Gq signaling in cardiomyocytes is being used for exploring cardiac function [87] and cardiac cell differentiation (in vitro/in vivo). Expression of melanopsin in retinal ganglion cells enabled the successful restoration of vision in blind mice [71]. Ectopic expression of melanopsin in hypothalamic orexin neurons, led to a long term activation of these neurons following blue light exposure [88].
Conclusion
Since its exciting discovery in 1998 in Xenopus laevis [19], melanopsin has transcended its earliest functional designation as a circadian pacemaker exclusively controlling non-visual functions of the eye. Recent research indicates the involvement of melanopsin in diverse functions ranging from image-forming vision, photo-allodynia, sleep, migraine pain sensations [89], mood disorders, anxiety and depression [2]. Future research will undoubtedly continue to provide further information on the diverse characteristics, functions and clinical relevance of this non-classical blue light-sensitive pigment. The successful use of melanopsin for the light-induced regulation of intracellular transcriptional/ translational functions in several in vivo and in vitro experiments is indicative that melanopsin might be successful as an independent optogenetic tool [17], or in combination with other photoactive proteins [90, 91], such as the channelrhodopsins [92, 93].
The successful marriage between optogenetics and synthetic biology may well provide programmable melanopsin-driven designer devices capable of influencing a wide variety of cellular transduction cascades through their sensitivity to blue light. These optogenetics-based gene/cell therapy approaches may ultimately involve identification of new molecular and circuit-level targets that will provide precise interventions for defined biochemical or cellular events [94]. An example is the successful attenuation of glycemic excursions, and the blue light-induced regulation of blood-glucose homeostasis through the melanopsin-triggered expression of the GLP-1 in the human type II diabetic mouse model [17]. Once the ethical and safety issues associated with such synthetic intracellular devices have been adequately addressed, we may then look forward to an "omics era", as described by Folcher and Fussenegger [95], where light-induced cell implants with integrated synthetic genetic circuits will routinely execute predictable therapeutic and metabolic functions.
Acknowledgement: I am thankful to Patrick Abramson for technical support for the preparation of the figures, and to Lawrence Brako for technical and editorial support, Morehouse School of Medicine, Atlanta GA.
References
1. Bailes HJ, Lucas RJ. Melanopsin and inner retinal photoreception. Cell Mol Life Sci. 2010; 67:99-111.
2. Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011; 34:572-80.
3. Sekaran S, Lupi D, Jones SL, Sheely CJ, Hattar S, Yau KW, Lucas RJ, Foster RG, Hankins MW. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005; 15:1099-107.
4. Fu Y, Zhong H, Wang MH, Luo DG, Liao HW, Maeda H, Hattar S, Frishman LJ, Yau KW. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Natl Acad Sci USA. 2005; 102:10339-44.
5. Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal color and irradiance and project to the LGN. Nature 2005; 433:749-54.
6. Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001; 4:1165.
7. Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003; 23:7093-106.
8. Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006; 497:326-49.
9. Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008; 27:1763-70.
10. Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010; 2:31ra33.
11. Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010; 8:e1000558. doi: 10.1371/journal.pbio.1000558.
12. Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 2010; 67:49-60.
13. Blasic JR Jr, Brown RL, Robinson PR. Phosphorylation of mouse melanopsin by protein kinase A. PLoS One. 2012; 7:e45387. doi: 10.1371/journal.pone.0045387.
14. Hatori M, Panda S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol. Med. 2010; 16:435-46.
15. Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS Biol. 2009; 7:e1000125.doi: 10.1371/journal.pbio.1000125.
16. LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 2012; 491:594-98.
17. Ye H, Daoud-El Baba M, Peng RW, Fussenegger M. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 2011; 332:1565-88.
18. Horner M, Weber W. Molecular switches in animal cells. FEBS Lett. 2012; 586: 2084-96.
19. Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998; 95:340-45.
20. Bellingham J, Whitmore D, Philp AR, Wells DJ, Foster RG. Zebrafish melanopsin: isolation, tissue localization and phylogenetic position. Brain Res Mol Brain Res. 2002; 107:128-36.
21. Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, Natesan A, Zatz M, Tosini G, Liu C, Korf HW, Iuvone PM, Provencio I. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J Neurochem. 2005; 92:158-70.
22. Frigato E, Vallone D, Bertolucci C, Foulkes NS. Isolation and characterization of melanopsin and pinopsin expression within photoreceptive sites of reptiles. Naturwissenschaften. 2006; 93:379-85.
23. Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000; 20:600-05.
24. Bellingham J, Chaurasia SS, Melyan Z, Liu C, Cameron MA, Tarttelin EE, Iuvone PM, Hankins MW, Tosini G, Lucas RJ. Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in non-mammalian vertebrates. PLoS Biol. 2006; 4(8):e254.
25. Pires SS, Shand J, Bellingham J, Arrese C, Turton M, Peirson S, Foster RG, Halford S. Isolation and characterization of melanopsin (Opn4) from the Australian marsupial Sminthopsis crassicaudata (fat-tailed dunnart). Proc Biol Sci. 2007; 274:2791-99.
26. Nayak SK, Jegla T, Panda S. Role of a novel photopigment, melanopsin, in behavioral adaptation to light. Cell Mol Life Sci. 2007; 64:144-54.
27. Do MT, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW. Photon capture and signaling by melanopsin retinal ganglion cells. Nature 2009; 457:281-87.
28. Benarroch EE. The melanopsin system: Phototransduction, projections, functions, and clinical implications. Neurology 2011; 76:1422-27.
29. Arendt D. Evolution of eyes and photoreceptor cell types. Int J Dev Biol. 2003; 47:563-71.
30. Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Arch. 2007; 454:805-19.
31. Hardie RC, Raghu P. Visual transduction in Drosophila. Nature 2001; 413:186-93.
32. Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008; 99:2522-32.
33. Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 2002; 298:2213-16.
34. Hannibal J, Hindersson P, Ostergaard J, Georg B, Heegaard S, Larsen PJ, Fahrenkrug J. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest Ophthalmol Vis Sci. 2004; 45:4202-09.
35. Hannibal J. Roles of PACAP-containing retinal ganglion cells in circadian timing. Int Rev Cytol. 2006; 251:1-39.
36. Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002; 22:RC191.
37. Wong KY, Graham DM, Berson DM. The retina-attached SCN slice preparation: an in vitro mammalian circadian visual system. J Biol Rhythms. 2007; 22:400-10.
38. Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, Cooper HM. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron 2007; 53:677-87.
39. Panda S. Multiple photopigments entrain the Mammalian circadian oscillator. Neuron 2007; 53: 619-21.
40. Whitmore D, Cermakian N, Crosio C, Foulkes NS, Pando MP, Travnickova Z, Sassone-Corsi P. A clockwork organ. Biol Chem. 2000; 381:793-800.
41. Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008; 6:e249. doi: 10.1371/journal.pbio.0060249.
42. Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007; 21:3866-71.
43. Dkhissi-Benyahya O, Coutanson C, Knoblauch K, Lahouaoui H, Leviel V, Rey C, Bennis M, Cooper HM. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci. 2013; [Epub ahead of print].
44. Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O'Hara BF. Role of melanopsin in circadian responses to light. Science 2002; 298:2211-13.
45. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002; 295:1065-70.
46. Tsujimura S, Ukai K, Ohama D, Nuruki A, Yunokuchi K. Contribution of human melanopsin retinal ganglion cells to steady-state pupil responses. Proc Biol Sci. 2010; 277:2485-92.
47. McDougal DH, Gamlin PD. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010; 50:72-87.
48. Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007; 47:946-54.
49. Kankipati L, Girkin CA, Gamlin PD. Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci. 2010; 51:2764-69.
50. Higuchi S, Hida A, Tsujimura S, Mishima K, Yasukouchi A, Lee SI, Kinjyo Y, Miyahira M. Melanopsin gene polymorphism I394T is associated with pupillary light responses in a dose-dependent manner. PLoS One. 2013; 8:e60310. doi: 0.1371/journal.pone.0060310.
51. Brown TM, Tsujimura S, Allen AE, Wynne J, Bedford R, Vickery G,Vugler A, Lucas RJ. Melanopsin-based brightness discrimination in mice and humans. Curr Biol. 2012; 22:1134-41.
52. Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 2003; 299:245-47.
53. Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P, Stolz S, Flockerzi V, Freichel M, Simon MI, Clapham DE, Yau KW. Melanopsin signaling in mammalian iris and retina. Nature 2011; 479: 67-73.
54. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology 2009; 116:1564-73.
55. Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol. 2007; 27:195-204.
56. Ishikawa H. Pupil and melanopsin photoreception. Nihon Ganka Gakkai Zasshi. 2013; 117:246-68; discussion 269.
57. Feigl B, Zele AJ, Fader SM, Howes AN, Hughes CE, Jones KA, Jones R. The post-illumination pupil response of melanopsin-expressing intrinsically photosensitive retinal ganglion cells in diabetes. Acta Ophthalmol. 2012; 90:e230-4. doi: 10.1111/j.1755-3768.2011.02226.
58. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005; 437:1257-63.
59. Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol. Rhythm. 2006; 21: 482-93.
60. Moore RY. Suprachiasmatic nucleus in sleep-wake regulation. Sleep Med. 2007; 3:27-33.
61. Fisher SP, Foster RG, Peirson SN. The circadian control of sleep. Handb Exp. Pharmacol. 2013; 217:157-83.
62. Hughes S, Hankins MW, Foster RG, Peirson SN. Melanopsin phototransduction: slowly emerging from the dark. Prog Brain Res. 2012; 199:19-40.
63. La Morgia C, Ross-Cisneros FN, Hannibal J, Montagna P, Sadun AA, Carelli V. Melanopsin-expressing retinal ganglion cells: implications for human diseases. Vision Res. 2011; 51:296-302.
64. Roecklein KA, Wong PM, Miller MA, Donofry SD, Kamarck ML, Brainard GC. Melanopsin, photosensitive ganglion cells, and seasonal affective disorder. Neurosci Biobehav Rev. 2013; 37:229-39.
65. Wehr TA, Skwerer RG, Jacobsen FM, Sack DA, Rosenthal NE. Eye versus skin phototherapy of seasonal affective disorder. Am. J Psychiatry. 1987; 144:753-57.
66. Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, Provencio I. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009; 114:279-85.
67. Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, Wisner KL, Nemeroff CB. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005; 162:656-62.
68. Roecklein KA, Wong PM, Franzen PL, Hasler BP, Wood-Vasey WM, Nimgaonkar VL, Miller MA, Kepreos KM, Ferrell RE, Manuck SB. Melanopsin gene variations interact with season to predict sleep onset and chronotype. Chronobiol Int. 2012; 29:1036-47.
69. Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin Proc Natl Acad Sci US A. 2005; 102:17816-21.
70. Sineshchekov OA, Govorunova EG, Wang J, Spudich JL. Enhancement of the long-wavelength sensitivity of optogenetic microbial rhodopsins by 3, 4-dehydroretinal. Biochemistry. 2012; 51:4499-506.
71. Koizumi A, Tanaka KF, Yamanaka A. The manipulation of neural and cellular activities by ectopic expression of melanopsin. Neurosci Res. 2013; 75:3-5.
72. Chow BY, Han X, Boyden ES. Genetically encoded molecular tools for light-driven silencing of targeted neurons. Prog Brain Res. 2012; 196:49-61.
73. Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM,Janak PH, Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 2011; 72:721-33.
74. Bacchus W, Fussenegger M. The use of light for engineered control and reprogramming of cellular functions. Curr Opin Biotechnol. 2012; 23:695-702.
75. Müller K, Weber W. Optogenetic tools for mammalian systems. Mol Biosyst. 2013; 9:596-608.
76. Chow BY, Boyden ES. Physiology. Synthetic physiology. Science 2011; 332:1508-09.
77. Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (λ max ≈ 479 nm) supporting activation of G(q/11) and G(i/o) signaling cascades. Proc Biol Sci. 2013; 280:20122987. doi: 10.1098/rspb.2012.2987.
78. Ausländer D, Fussenegger M. Optogenetic therapeutic cell implants. Gastroenterology 2012; 143:301-6.
79. Parsons GB, Souza DW, Wu H, Yu D, Wadsworth SG, Gregory RJ, Armentano D. Ectopic expression of glucagon-like peptide 1 for gene therapy of type II diabetes. Gene Ther. 2007; 14:38-48.
80. Ahrén B. Emerging dipeptidyl peptidase-4 inhibitors for the treatment of diabetes. Expert Opin Emerg Drugs. 2008; 13:593-607.
81. Ahrén B. Clinical results of treating type 2 diabetic patients with sitagliptin, vildagliptin or saxagliptin-diabetes control and potential adverse events. Best Pract Res Clin Endocrinol Metab. 2009; 23:487-98.
82. Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care 2010; 33:428-33.
83. Kielgast U, Holst JJ, Madsbad S. Treatment of type 1 diabetic patients with glucagon-like peptide-1 (GLP-1) and GLP-1R agonists. Curr Diabetes Rev. 2009; 5:266-75.
84. Ausländer S, Ausländer D, Müller M, Wieland M, Fussenegger M. Programmable single-cell mammalian biocomputers. Nature 2012; 487:123-27.
85. Hernández RM, Orive G, Murua A, Pedraz JL. Microcapsules and microcarriers for in situ cell delivery. Adv Drug Deliv Rev. 2010; 62:711-30.
86. Ausländer S, Fussenegger M. From gene switches to mammalian designer cells: present and future prospects. Trends Biotechnol. 2013; 3:155-68.
87. Beiert T, Bruegmann T, Kilgus C, Fleischmann BK, Sasse P. Light induced Gq signaling in cardiomyocytes using optogenetics. Acta Physiologica 2012; 689:73.
88. Tsunematsu T, Tabuchi S, Tanaka KF, Boyden ES, Tominaga M, Yamanaka A. Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behav Brain Res. 2013; pii: S0166-4328(13)00292-1. doi: 10.1016/j.bbr.2013.05.021
89. Johnson J, Wu V, Donovan M, Majumdar S, Rentería RC, Porco T, Van Gelder RN, Copenhagen DR. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci USA. 2010; 107:17374-78.
90. Fenno L. Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev NeuroSci. 2011; 34:389-412.
91. Wang X, Chen X, Yang Y. Spatiotemporal control of gene expression by a light-swtichable transgene system. Nat Methods. 2012; 9:266-269.
92. Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AB, Bamberg E, Hegemann P. Channelrhodopsin-1: A light gated proton channel in green algae. Science. 2002; 296: 2395-2398.
93. Nagel G. Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2: A directly light-gated cation-selective membrane channel. PNAS. 2003; 100:13940-13945.
94. Chow BY, Boyden ES. Optogenetics and translational medicine. Sci Transl Med. 2013; 5:177ps5.doi:10.1126/scitranslmed.3003101.
95. Folcher M, Fussenegger M. Synthetic biology advancing clinical applications. Curr Opin Chem. Biol. 2012; 16:345-54.
11/12/13