APPLIED PHOTOSYNTHESIS for BIOFUELS PRODUCTION
Michael Seibert
National Renewable Energy Laboratory, Golden, CO 80401
mike.seibert@nrel.gov
Background
The earth receives 176,000 terawatts of incident primary power, while the rate of human world energy consumption is about 13.5 terawatts or less than 0.01% of the solar resource. While solar energy is disperse, nevertheless it represents a daily average energy equivalent of 20 kilotons of TNT per 1.5 square miles of the earth's surface. Nature has provided a very useful storage process for capturing this light energy, namely photosynthesis. In fact photosynthesis on earth stores over 10 times the energy each year than that used currently by all humanity. Throughout human history, from a practical perspective, photosynthesis has provided us with the oxygen we breathe, the food we eat, the materials for our houses, the clothes we wear, and the fuel that we use. With the industrial revolution, stored photosynthate in the form of wood supplied the largest proportion of the fuel energy (1740-1885) used to run the machines and factories that represented the new industrial might of the rapidly developing world. Fossil fuels (products of ancient photosynthesis) first came into the US energy picture around 1850, with coal overtaking wood in 1885, oil overtaking coal in 1950, and natural gas overtaking coal in 1955. Nuclear power entered the world's energy mix in the early 1950's. It currently represents about 8.4% of the energy used in the US today, and almost all of that is for the generation of electricity. Realistically, however, at current rates of growth in energy use, fossil fuels and nuclear will last for about another century, and then will have to be replaced.
The oil crises of 1973-1974 and 1979 were wake-up calls for the energy problems to come, but efforts to address these problems were short term, and mostly limited to an emphasis on energy conservation (increasing the energy efficiency of our cars, buildings, homes, and industrial processes). Since the mid 1970s, the use of wood, forest waste, and wood products has made a small resurgence in the US energy picture, particularly in the forest products industry and for electrical power production. Nevertheless, some renewable energy research (i.e., biofuels, wind, photovoltaics [PV], geothermal, ocean thermal energy conversion [OTEC], wave power, biohydrogen, and solar thermal technologies that produced electricity, heat, and liquid fuels from biomass) was supported, though intermittently, throughout the 1980s and 1990s. The results of this work, including the commercialization of cost-competitive wind power, significant progress towards the commercialization of PV, and the extensive use of biomass along with low and high head hydropower now adds up to about 7% of the 2007 US primary energy inventory.
Nevertheless, the world is currently using energy at a rate of over 200 million barrels of oil equivalent per day, but demand in 2050 could be as high as 30 terawatts (a large file), or almost 2.5 times current usage. To put this in perspective, Albert Bartlett calculated that at the 1978 prevailing growth rate in oil consumption (7.04%), it would take only 342 years to consume 6.81 x 1021 barrels of oil, which is the entire volume of the earth! Currently oil is being used at almost 1000 gallons per second, and it is estimated that world oil production will peak anywhere from 2009 to 2020. Gasoline prices at the pump, until recently, were over $4.00 a gallon in the US (to the amusement of those in Europe and Japan). Currently the price of gasoline is back down to the $1.50-1.80 level, but the price of oil could go back up to the recent high of $147 per barrel or higher at any time. Furthermore, levels of CO2 in the atmosphere (387 ppm in the spring of 2008) could reach 600 ppm by 2035, if a "business as usual" attitude continues. All of this adds up to serious potential economic and environmental problems around the world.
This module will briefly examine how photosynthesis is being harnessed for energy applications now, and how it might be developed for even greater practical impact in the near future. The Energy Independence and Security Act of 2007 increased the Renewable Fuels Standard in the US to 36 billion gallons of Biofuels by 2022 (15 billion from corn-grain ethanol, 1 billion from biodiesel, and 21 billion from advance biofuels, which in addition to cellulosic ethanol might include algal biodiesel, biohydrogen, alkanes, biobutanol, Fischer-Tropsch (F-T) liquids, methane, and others). All of this will have to be produced by photosynthesis-driven processes. The US Departments of Agriculture and Energy determined in 2005 that the country could sustainably generate 1.3 billion dry tons of biomass feedstock per year, enough to displace 30% of the gasoline consumption at the time with biofuels. In reality, there are two major approaches to producing biofuels, i.e., by either indirect or direct conversion processes. The former involves using photosynthesis to grow biomass in a first step, and then in a second step converting the biomass to the final product, be it fuel, power, or a useful byproduct (first- and second-generation processes as outlined below). The latter seeks to harness photosynthesis to produce the final product directly by single-step (third-generation) processes. Both these approaches will be important for meeting US energy goals.
First Generation Biofuels
Wood-Fueled Power. Recently, the terms biomass and biofuels have become very popular, but the historical burning of wood (and more recently highly compacted sawdust [wood] pellets) for residential heating and process heat in the forest products industry has continued, particularly in the northeast, southeast, and the mountain regions of the US. Wood pellets are particularly attractive in residences, since they are very dense, have water content below 10%, can be transported more economically than firewood, store well, have about 83% of the energy content of bituminous coal, and are amenable to automated feeding. About 35 years ago, significant amounts of electrical power started to be generated in the US from biomass, and in 2007 over 10 billion KW-hours of energy were produced (Figure 1). The Energy Information Administration has estimated that of the 590 million wet tons of wood biomass available in the US on an annual basis, 20 million (enough to supply about 3 gigawatts of capacity out of 1,075 gigawatts of total US capacity in 2006) are available at prices of $1.25 per million Btu or less.

Figure 1. A 50-MW electrical power-generating station in Burlington, Vermont, uses wood as a renewable fuel. [PIX 06814 from the National Renewable Energy Laboratory (NREL) picture collection.]
Corn-Grain Ethanol. Because of Federal farm subsidies for corn production and refinery subsidies for blending ethanol with gasoline, corn grain ethanol production has seen recent remarkable growth. Ethanol is produced by traditional fermentation processing of the starch extracted from the kernels of field corn ears. As of October 2008, there were 178 operating ethanol plants (Figure 2), and 26 more under construction in the US with an operating capacity of 10.955 billions gallons of fuel-grade ethanol (the US uses about 140 billion gallons of gasoline per year). In 2007, about 27% of the total corn crop (13.1 billion bushels) was used for ethanol production, and the total corn acreage was up 15% from 2005. Recently, a number of issues have arisen that will ultimately place limits on the amount of corn grain ethanol biofuel produced in the US. These include the huge amount of land area required for fuel production (acerbating the food versus fuel debate in terms of increases in food prices); the amount of fossil energy needed to produce fuel ethanol (how much is the energy gain in the ethanol compared to the amount of fossil fuel used during all aspects of ethanol production); increases in soil erosion; increases in fertilizer, herbicide, and pesticide pollution; increases in water usage; and degradation of wildlife habitat. The price of ethanol, of course, depends on the price of corn. At $2/bushel, ethanol can be produced at $1.10-$1.50/gallon; at $8/bushel, it is as high as $3.25/gallon.

Figure 2. Field corn (left) and the Front Range Energy plant (right) located in Windsor, CO. The plant processes corn grain, and it produces about 40 million gallons of ethanol and 396,000 tons of wet distillers grain as a by-product annually. [PIX 10477 & 15042]
Biodiesel from Plant Oils. A variety of vegetable oils can be used as a substitute for diesel from petroleum. Up until the 1920s, diesel engines (invented in the 1893) actually ran on non-transesterified peanut oil, which is not classified as biodiesel (a mono-alkyl ester). The process of transesterification was patented in Belgium in 1937, although the chemistry was demonstrated in 1853, long before the invention of the diesel engine. It involves the catalytic "alcoholysis" of vegetable oils using ethanol or methanol to remove the glycerol from triacylglycerol (TAG, mostly neutral lipids) by replacing it with short linear alcohols. In the 1920s, diesel engine manufacturers altered their engines to utilize petrodiesel rather than vegetable oil. The former had a lower viscosity, and was much cheaper. Thus, the vegetable oil fuel industry was essentially eliminated, except for a brief period during WWII. Within the last 25 years, environmental concerns and a decrease in the price differential made biodiesel a growing alternative to fossil-derived diesel, particularly in Europe and South Africa.
Currently biodiesel is made from soybean and rapeseed oils, as well as from mustard, flax, sunflower seed, and palm oils, but it can also be made from waste vegetable oil, animal fats, and Jatropha oil in the near future. In late 2006, soybean oil accounted for over 90% of the biodiesel produced in the USA, or 870 million gallons of capacity from 114 plants in production or under construction. For comparison, the country uses about 66 (40 of it for on road use) billion gallons of petroleum diesel each year. Unfortunately, the yield of biodiesel from soybean oil is only the equivalent of 40-50 gallons per acre, which is not a sustainable solution for our fuel needs. Nevertheless, first generation processes have been used to produce jet fuel, and Air New Zealand reported in December 2008 that it successfully flight-tested a blend of standard jet fuel and synthetic jet fuel made from Jatropha oil. Finally, there is a $1/gallon blenders (not production) tax credit for biodiesel.
Currently about 3.7% of the 101.6 quads (quadrillion BTUs or 1.07 x 1020 joules) of US primary energy used in 2007 came from the various biomass sources outlined above.
Second Generation Biofuels
Recent economic and social issues associated with first-generation biofuels, particularly the issues discussed above, have stimulated new ideas for the more efficient production of bioethanol. Currently two major research directions are being pursued: (a) lignocellulose biomass deconstruction to simple sugars such as glucose and xylose, and subsequent fermentation to bioethanol or other products and (b) thermochemical conversion of biomass to fuels and chemicals. Cellulosic biomass sources that are being developed as feed stocks include corn cobs, stover (dried stalks) and leaves; switchgrass; wood chips from short rotation crops such as cottonwood (Figure 3); Jatropha (tropics); and Miscanthus x giganteus (an Asian grass). Miscanthus can grow up to 12 feet tall on marginal land with little water, is cold tolerant, and is grown in Europe as an energy source. Compared to first generation ethanol technology, which reduces net greenhouse gas emissions on average by 18%, cellulosic ethanol is far superior, with projected reductions of 88%, though the exact numbers depend on the sources of heat and power used in the mill, and on direct and indirect changes in agricultural land use with the expansion of biofuels production.

Figure 3. Close-up of switchgrass plants 210 cm in height (left), and a short-rotation Cottonwood tree farm (right). [PIX 06690 & 08364]
Biomass Deconstruction and Fermentation. This approach, which can trace its history back to Germany in the late 1890's, was used in Europe and the USA during both World Wars. It first involves pretreatment (Figure 4) to fractionate, solubilize, hydrolyze, and separate biomass into cellulose, hemicellulose, and lignin components. Various processes can do this, but a cost-effective process is still under development using dilute acids, SO2, alkali, alkaline peroxide, wet-oxidation, ammonia fiber expansion, steam explosion, and other approaches to separate the biomass components. The aim is to reduce the size of the biomass, and open up the biomass structure (stabilized by the presence of lignin in many plant substrate materials) to liberate and decrystalize the cellulose. Chemical or enzymatic reactions are then used to hydrolyze the cellulose and produce glucose for subsequent fermentation to ethanol. Both concentrated acid treatment and the use of cellulases have been employed to hydrolyze the cellulose. Acid treatment has the advantage of releasing soluble xylose, but the disadvantage of producing toxic degradation products (furfural and hydroxymethyl furfural), which can affect down-stream processing, and the acid must be removed from the sugar stream.
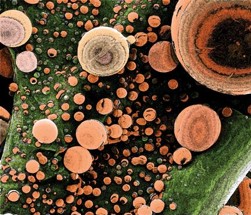
Figure 4. The thermal pretreatment of lignocellulosic biomass disperses lignin, which normally acts as a biomass "glue", into droplets of varying sizes. In this image from NREL's Biomass Surface Characterization Laboratory, the lignin droplets are shown in false-color orange, and range in diameter from 50 to 10,000 nanometers. The remaining cellulose component can be hydrolyzed to sugar for fermentation to ethanol. [PIX 15105]
Enzyme hydrolysis using engineered cellulases from the fungus, Trichoderma reesei (Figure 5), as well as engineered xylanases and hemicellulases are also being developed and used.

Figure 5. NREL scientists use a robotic deck to quickly culture genetic variants of fungal genes for producing enzymes, and then screen them to identify variants with the most promising characteristics. This is a temperature-controlled microplate stacker for storing microplates that will be transferred by robotic arm to the liquid handling workstation for assay and analysis. [PIX 1281]
To facilitate the release of cellulose and hemicellulose, enzymes that can degrade lignin to facilitate the release of these polymers for sugar production might be obtained from white rot fungus (Phanerochaele chrysosporium). Once sugars (mostly glucose), which depending on the biomass source can also include xylose and arabinose (5-carbon sugars from the hemicellulose fraction of the biomass), are generated from the hydrolysis process, ethanol is produced by fermentation (Figure 6). Yeast (Saccharomyces cerevisiae) and bacteria, including Zymomonas mobilis and Escherichia coli, have been used in this part of the process, depending on the available sugars. Recently yeasts have been developed that can ferment multiple sugars (e.g., Pichia stipitis can ferment 6- and 5-carbon sugars, including xylose), and they are attractive because they can grow at low pH (to avoid bacterial contamination) and are tolerant to inhibitors and high ethanol concentrations. Some bacteria, including Clostridium thermocellum, can produce a suite of more than 25 enzymes that can simultaneously degrade biomass and ferment the resulting sugars (termed consolidated bioprocessing). But they also produce other fermentation products such as lactate and acetate, which can lower the yield of ethanol. R&D is already underway to minimize these ancillary pathways.
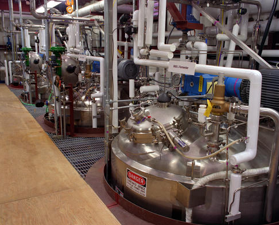
Figure 6. The process development unit (PDU) located at NREL can make available 9000-liter biomass-to-ethanol fermenters to outside users under partnership arrangements. [PIX 00945]
Another approach to the biomass recalcitrance problem (i.e., the difficulty in deconstructing biomass) is to develop more easily digestible biomass sources. There are projects at the US Department of Energy's Bioenergy Science Center to examine this possibility by (a) mapping the distribution of cellulose, hemicellulose, and lignin contents in various biomass samples, (b) determining why certain plants are more easily digestible, and (c) manipulating high yield biomass sources for improved digestibility. One advance in this area was a recent report out of Penn State showing that the genetic insertion of a protein into lignin results in a polymer with the normal strength of lignin, but can be attacked by enzymes that degrade proteins (an easier job than using enzymes that attack lignin directly).
In all the above cases, the ethanol, once produced, is concentrated by distillation. Currently there are operating cellulosic ethanol pilot plants in Salamanca (Spain), Saskatchewan (Canada), and in China (China Resources Alcohol Corporation). To see a list of US plants CLICK HERE.
This same general fermentation approach can be used to make other potential fuels such as biobutanol (a DuPont/British Petroleum consortium is already building a pilot plant in the UK), isobutanol (it can be catalytically converted through isobutylene to octane, aromatics, and constituents of gasoline), alkanes, H2, isopropanol, and other long chain alcohols (they are less corrosive, more gasoline-compatible fuels than ethanol). The approach is to identify or engineer organisms that can be used during the fermentation stage after the sugar in produced from biomass. Butanol, for example, contains about 90% of the energy of gasoline, whereas ethanol contains about 70%.
Finally, a number of companies are developing fermentation processes for producing products other than fuels. Some examples are 1,3-propanediol (an intermediate in the production of polymers), polyethylene, amino acids, polylactic acid (a biodegradable plastic), and perhaps even starch. At this point, the process substrates are corn sugar, glycerin (a by-product from biodiesel production), and cane sugar. However, future processes would make use of sugars derived from deconstructed biomass.
Thermochemical Processing. Thermochemical approaches can also be used to produce ethanol. Biomass, wood chip, or biomass waste is first gasified to produce a mixture of CO, CO2, and H2 (synthesis gas or "syngas"), which supplants the need to produce sugars. Syngas is then feed to Clostridium bacteria, which ferment synthesis gas and produce ethanol. Alternatively, the synthesis gas can be catalytically converted to ethanol, higher alcohols, and other liquids, such as gasoline and F-T diesel through an additional thermochemical catalytic process. Other options are also possible. For example, Black liquor containing dissolved wood lignin and pulping chemicals from the paper industry, might be gasified to syngas (while recovering the chemicals), and then converted to dimethyl ether (DME). DME can be used as a replacement for liquefied petroleum gas (LPG), or a substitute for diesel fuel. However, there are gasification reactor material issues that must be addressed, and the use of DME would require the adaptation of current diesel engine fuel systems. Nevertheless, if every paper mill in the US were adapted to produce DME, 7.5 billion gallons of fuel equivalent could be produced every year, but, as of 2008, US paper mills are closing due to low cost foreign competition. Another approach might be to convert the syngas to synthetic diesel fuel. The trade-offs are that "bioDME" produces over 65% more travel miles per acre of biomass input, and the highest greenhouse reduction for the lowest cost; but synthetic diesel could be blended with fossil-derived diesel, distributed by the current distribution network (DME would need its own dedicated network), is a little cheaper to produce than DME, and has a higher energy density.
Besides gasification carried out in the presence of oxygen, biomass can also be pyrolyzed at lower temperature in the absence of oxygen. Pyrolysis is the process that turns coal into coke used for making steel, or turns biomass into fine charcoal to make specialty steels. The products of wood, biomass crop, or organic waste pyrolysis are H2, methane, tars, char and "bio-oil" (Figure 7). The bio-oil is not a synthetic diesel fuel, which can't be produced by pyrolysis at this point, but it can be used as a boiler fuel after removing useful chemicals that can be used as food additives or in the pharmaceuticals industry. In the presence of water, many feedstocks can be pyrolyzed to generate a bio-oil fuel resembling light crude oil, which can be refined by traditional methods. One approach being examined by industry is to produce the bio-oil (also called bioliq) by pyrolysis in small regional plants, and then transport the energy-dense liquid to a central facility (there are cost advantages) where the bioliq would be converted to syngas in the presence of O2 and heat. Additional processing can convert the syngas to higher grade products such as methanol, H2, and synthetic diesel. Alternatively, the bio-oil can be treated with H2 and converted by catalytic reactions to gasoline and diesel-like fuels.

Figure 7. Biomass (wood) chips can be pyrolyzed to a dark brown, viscous liquid. In this application process heat is applied rapidly to the biomass in the total absence of O2, and the process is typically carried out in fluidized beds with an inert gas used as the fluidizing medium. Liquid yields of up to 70 weight % have been demonstrated under optimum conditions. The bio-oil liquid can be used in fuel applications, or in making useful chemical compounds. [PIX 13194]
Algal Biodiesel. Another possibility for advanced biofuels under development is the use of algae to produce lipids for biodiesel heterotrophically by fermenting carbohydrates. The sugar could come from traditional sources such as sugar cane or corn, but eventually a more cost effective and environmentally sound course would be to use sugars produced by the deconstruction of biomass, as outlined above. Several companies are exploring this option. However, one San Francisco-based company (e.g., Treehugger) reports a production alga that can store as much as 75% oil per gram of cell dry weight, and can currently ship algal oil in 500-gallon lots.
Third Generation Biofuels
To demonstrate the potential of an advanced direct photoconversion process (e.g., direct photosynthetic production of biohydrogen or biodiesel from water, CO2, and sunlight) compared to an indirect one (e.g., biomass production in a first step and then conversion to a fuel such as ethanol in a second as discussed above), Figure 8 shows the area of the US that would have to be covered with photobioreactors in order to displace the current amount of gasoline used in the country (about 140 billion gallons per year). The assumptions are an algal solar-to-H2 conversion efficiency (using the average yearly US solar irradiance) at the current national solar conversion efficiency goal of 10% (a very challenging one), and utilization of the H2 in fuel-cell-powered vehicles getting 60 miles per gallon of gasoline energy equivalent. Also shown is the bioreactor area required at 2% photosynthetic conversion efficiency (likely achievable at some point in the future) to produce enough algal biodiesel to displace all on-road fossil diesel (about 40 billion gallons per year) used in the US on a volume basis. For comparison, the land areas required to displace all US gasoline with ethanol are also indicated, if the ethanol were produced from (a) corn grain (assuming that the 23,750 square miles of corn devoted to ethanol production in 2006 displaced 2.4% of the gasoline used in the country that year on an energy basis), or (b) cellulose (assuming that the maximum achievable production for switchgrass in the US is 20 dry tons per acre-year [personal communication, Kelly Ibsen]). Realistically no one technology will produce all the fuel used in the USA, but direct conversion processes hold much promise for the future, especially in terms of land utilization efficiency, if R&D can lead to cost-competitive, sustainable processes.
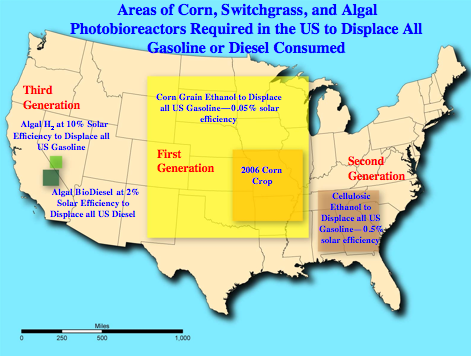
Figure 8. Areas of corn and switchgrass fields or algal photobioreactors required to displace all gasoline or diesel used for transportation applications in the US. [Copyright 2008, Midwest Research Institute. Created with U.S. Government funding.]
Algae (including microalgae and cyanobacteria) represent a diverse, yet highly specialized group of water-splitting, photosynthetic microorganisms that live in many ecological habitats, such as fresh, brackish, marine, and hypersaline waters. An advantage is that they don't need soil, and grow over a range of temperatures, pHs, and nutrient conditions. Furthermore, they grow very quickly, can be harvested daily if necessary, and can be cultured in ponds or closed photobioreactors. Over 40,000 species have been identified, with many more yet to come. Algae are classified as cyanobacteria (Cyanophyceae), green algae (Chlorophyceae), diatoms (Bacillariophyceae), yellow-green algae (Xanthophyceae), golden algae (Chrysophyceae), red algae (Rhodophyceae), brown algae (Phaeophyceae), and "picoplankton" (Prasinophyceae and Eustigmatophyceae). Several additional divisions and classes of unicellular algae have been described, and details of their structure, biology, and physiology are available. Thousands of species and strains of these algal taxa are currently maintained in culture collections and by scientists throughout the world (e.g., List of Algal Culture Collections and Culture Collections in the World), but there are also several new efforts to discover new strains in the wild that are suitable for biofuels applications.
Considering the diversity of organisms potentially available in the environment, algae could be very important in a future mix of third-generation biofuels technologies as indicated in Figure 9. The figure shows that they can produce important intermediate metabolites that can be directly converted biologically to many different types of fuels independent of a biomass stage as in first and second generation processes.
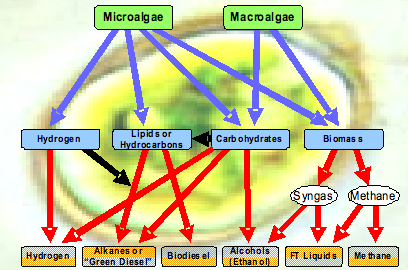
Figure 9. Microalgae, functioning as photocatalysts, can potentially use photosynthesis to directly convert water, CO2, and sunlight to a variety of different fuels without going through an intermediary biomass stage (thanks to Al Darzins and Eric Jarvis at NREL for this graphic).
Algal Biohydrogen Production From Water. Certain photosynthetic microbes, including algae and cyanobacteria, can produce H2 from the world's most plentiful resources in the following reactions: 2H2O + light energy -> O2 + 4H+ + 4e- -> O2 + 2H2. The first recorded observation of this was reported over a hundred and ten years ago, when a natural bloom of Anabaena after being placed in a glass jar, started to produce H2. Hydrogen uptake and production were the first reports of H2 metabolism in a green alga (Scenedesmus obliquus) in the early 1940's.
Two distinct light-driven, H2-photoproduction pathways have been described in green algae, and there is evidence for a third, light-independent, fermentative H2 pathway coupled to starch degradation. All pathways have the reduction of ferredoxin (FD, Figure 10) in common as the primary electron-donor to a hydrogenase. Hydrogenases are enzymes that can reduce protons and release molecular H2. The major types of hydrogenases contain either iron ([FeFe]-hydrogenases, which generally are H2-evolving) or both nickel and iron ([NiFe]-hydrogenases, which are generally H2-uptake enzymes) in their active sites. Plants do not contain hydrogenase genes. (Additional information about these O2-sensitive enzymes can be found below.) The light-driven pathways can either use water as the substrate (employing both photosystems II and I) or NADH from the glycolytic breakdown of stored carbohydrate (employing only photosystem I) to product H2. Rather than utilizing the light-driven reduction of FD, the dark, fermentative pathway may also involve a pyruvate-ferredoxin-oxidoreductase (PFOR) enzyme, similar to those found in many anaerobic systems. Although PFOR-catalyzed pyruvate oxidation/FD reduction in the C. reinhardtii fermentative H2-production pathway is not proven absolutely under dark, H2-producing conditions, a PFOR gene is up-regulated in C. reinhardtii. This suggests that PFOR might provide the link between fermentative carbon dissimilation and H2 production. Up until the beginning of the 21st century, prospects for using algae to produce H2 as a fuel (in actuality H2 is classified as an energy carrier since it is not found naturally in the environment, and must be produced) were academic, despite a number of reports on the production of H2 gas using the nitrogenase function of cyanobacteria (prokaryotic, blue-green algae). This latter work, however, waned in the 1990's with the realization of limitations in the ultimate projected light conversion efficiencies obtainable by this approach.
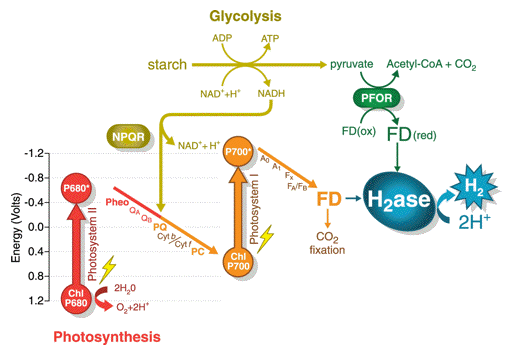
Figure 10. Algae exhibit 3 different pathways for H2 production. Two are driven by light and the third occurs in the dark. Either water or starch can be the electron donor. Carbon is fixed under normal photosynthesis with water as the donor, but the electron acceptor is switched at the level of ferredoxin (FD) from carbon dioxide to protons under conditions that lead to H2 production. (thanks to Prof. M. Posewitz, Colorado School of Mines for the drawing)
In 2000, a team at NREL and the University of California, Berkeley, reported that a relatively large amount of H2 could be produced for up to 4 days by the green alga, C. reinhardtii, after imposition of sulfur-deprivation stress. This technical advance rekindled interest around the world in H2-producing phototrophs and [FeFe]-hydrogenases. Since that time, the mechanism explaining the phenomenon of sulfur stress has been elucidated; and it involves the co-occurrence of aerobic photosynthesis, anaerobic fermentation, and respiration. Specifically, since hydrogenase enzymes (at least non-H2-sensing hydrogenases) are deactivated by O2 and since algae produce O2 as a by-product of photosynthesis, it is not possible at this point for them to produce H2 for long when O2 is present. However, when sulfate is removed from the medium of a mature algal culture, photosystem II (PSII) activity (see Figure 10), and consequently O2-evolution capacity in the algae, decreases because the PSII repair process is affected. When the O2-evolution rate in the culture falls below the O2-uptake rate of respiration, hydrogenase synthesis and subsequent enzyme activation occurs, and H2 starts to bubble out of the culture shortly thereafter (Figure 11). While this works in that a volumetric amount of H2 can be produced, a big price is paid with respect to the maximum energy conversion efficiency of the process. In order to evolve H2 in this manner, one has to sacrifice over 90% of the maximum rate of photosynthesis. Nevertheless, recent improvements in the rates of H2 production and the time over which H2 can be produced have been reported both in wild-type and mutant organisms. Currently, algal suspension cultures can produce up to one volume of H2 per two volumes of culture over a period of about 4 days. Furthermore, low light level, laboratory-scale energy conversion efficiencies in terms of light energy stored as H2 have surpassed 1% in wild-type C. reinhardtii cultures immobilized in alginate films (which can partially protect the O2-sensitve hydrogenase enzyme during H2 production). Furthermore, algae immobilized on glass fibers have produced H2 continuously for up to 90 days. Recent reports have also demonstrated that certain algal mutants exhibit increased rates of H2 production compared to their parental strains. Since all of these advances have been reported in different bioreactor systems, the challenge will be to demonstrate all of these improvements in one system and then to further improve the biology of that system.

Figure 11. Laboratory scale photobioreactors located in the author's laboratory at NREL, which have been used to monitor a number of different physiological parameters in sulfur-deprived Chlamydomonas reinhardtii, a green soil alga. This was the organism of choice since more is know about this species than any other strain of green alga, and a sequenced genome is available. In this simple demonstration, H2 is being collected in an inverted graduate cylinder (upper center right) by the displacement of water. [PIX 08741]
Four biological challenges limiting biohydrogen production in algae have been identified as, (a) the O2 sensitivity of hydrogenases, (b) competition for photosynthetic reductant at the level of ferredoxin, (c) regulatory issues associated with the over production of ATP, and (d) inefficiencies in the maximum utilization of solar light energy. Many laboratories around the world are addressing these challenges by, (a) engineering hydrogenases to improve the enzyme's tolerance to the presence of O2, (b) identifying metabolic pathways that compete with hydrogenases for photosynthetic reductant by genomics approaches, and engineering their down-regulation during H2 production, (c) engineering the photosynthetic membrane to significantly decrease the efficiency of photosynthetic-electron-transport-coupled ATP production (not depicted in Figure 10; ATP is required for carbon fixation, but not for hydrogenase-coupled H2 production), and (d) engineering the photosynthetic antenna pigment content to maximize the amount of solar light that can be used effectively in a photobioreactor. If all of the research challenges can be over come, H2-cost projections developed by the US Department of Energy suggest that biohydrogen could compete with gasoline at about $2.50 a kg (a gallon of gasoline contains the energy equivalent of about a kg of H2).
Recently, researchers have begun to re-examining the prospects for using cyanobacteria to produce H2. These studies are making use of bidirectional, [NiFe]-hydrogenases that are found in some of these organisms. While many of the same challenges identified in eukaryotic algae are also inherent in cyanobacteria, the advantages of working with these prokaryotic organisms are that they are more easily engineered than eukaryotic algae, and have more O2-tolerant hydrogenases. On the other hand, the [FeFe]-hydrogenases, found in algae, are better catalysts than the [NiFe]-hydrogenases found in cyanobacteria.
All H2-producing systems face the same non-biological challenge of efficient storage capability. While H2 has the highest energy density in terms of weight of any fuel, it compares poorly with liquid fuels on volumetric energy density. There are many projects in the US exploring the use of chemical-hydride, metal-hydride, and sorption technologies to enhance the ability to store H2 for transportation applications, where the size of the fuel tank is critical. Notably, a recent Lawrence Livermore National Laboratory report of a high-pressure, cryogenic storage, proof-of-concept technology that can run a hybrid-electric vehicle for up to 650 miles on a tank of H2 (it can fit into the back of the vehicle) is encouraging.
Other areas of investigation for the future that researchers are starting to examine, include the application of biological knowledge of photosynthesis and hydrogenase structure/function to develope biohybrid systems (those employing biological and synthetic components), and ultimately, totally artificial photosynthetic systems that mimic the fuel-producing processes of photosynthetic organisms.
Algal Biodiesel. Algae can survive and grow over a wide range of environmental conditions due in part to the tremendous diversity and occasional unusual pattern of cellular lipids, as well as due to their ability to modify lipid metabolism efficiently in response to changes in environmental conditions. These lipids include neutral lipids, polar lipids, wax esters, sterols, and hydrocarbons, as well as prenyl derivatives such as tocopherols, carotenoids, terpenes, quinones, and phytylated pyrrole derivatives (e.g., chlorophylls), but others are also possible. Notably oleaginous algae have been recognized, isolated, and evaluated for lipid content for over 60 years,
Under optimal growth conditions, algae synthesize fatty acids principally for esterification into glycerol-based membrane lipids, which constitute about 5 to 20% of their dry cell weight (DCW). Fatty acids include medium-chain (C10-C14), long-chain (C16-C18), and very long-chain (>C20) species. Major membrane lipids include glycosylglycerides (monogalactosyldiacylglycerol, digalactosyldiacylglycerol, and sulfoquiinovosyldiacylglycerol), enriched in chloroplasts, and phosphoglycerides (phosphatidylethanoamine and phosphatidylglycerol), found mainly in the plasma membrane and many endoplasmic membrane systems. The major constituents of the membrane glycerolipids are various kinds of fatty acids that are polyunsaturated and derived through aerobic desaturation and chain elongation from the 'precursor' fatty acids, palmitic and oleic acids.
Many algae redirect their lipid biosynthetic pathways under unfavorable environmental or stress conditions towards the formation and accumulation of neutral lipids. These are mostly in the form of triacylglycerol (TAG), which is similar in chemical composition to oils and fats produced in higher plants, particularly oilseed crop plants. TAGs do not perform a structural role (like the glycerolipids found in membranes), but instead serve as a storage medium for carbon. However, the TAG biosynthesis pathway in algae may also play a more active role in stress response than just carbon storage (e.g., lipids could act as a sink for NADPH). Unlike higher plants, where individual classes of lipid may be synthesized and localized in specific cells, tissues or organs, many different types of lipids occur in unicellular algae. TAGs after synthesis are deposited in densely packed lipid bodies located in the algal cytoplasm, though the formation and accumulation of lipid bodies also occur in the inter-thylakoid space (called plastoglobuli) of the chloroplast in the green alga, Dunaliella bardawil.
Hydrocarbons are also found in algae, but in quantities of less than 5% DCW. Only the colonial green alga, Botryococcus braunii, has been shown to produce, large quantities (up to 80% DCW) of very long-chain (C23-C40) hydrocarbons under adverse environmental conditions. These hydrocarbons are similar to those found in petroleum, and many researchers have suggested them as potential feedstocks for biofuels and biomaterials production. These will not be discussed further since little progress in adapting the organism for making large amounts of material has been reported.
It has long been argued that algal cultures could be employed as cell factories to produce oils and other lipids for biofuels and other biomaterials. The US Department of Energy in fact sponsored such a program (the so-called Aquatic Species Program) from 1978 to 1996 (it was terminated due to low oil prices and declining federal budgets at the time). However, the potential advantages of using algae to generate feedstocks for biofuels and biomaterials applications include their ability to, (a) synthesize and accumulate large quantities of storage neutral lipids/oil (20-50% DCW), (b) grow rapidly (e.g., 1 to 3 doubling times per day), (c) thrive in saline water, brackish water, or coastal seawater which are readily available, (d) tolerate marginal lands (e.g., desert, arid- and semi-arid lands), which are not suitable for conventional agriculture, (e) utilize nutrients such as nitrogen and phosphorus from a variety of wastewater sources (e.g., agricultural runoff, concentrated animal feed operations, and industrial and municipal wastewaters, thus providing the additional benefit of wastewater bioremediation) for growth, (f) capture CO2 from flue gases emitted from fossil fuel-fired power plants and other sources, thereby reducing the emissions of a major greenhouse gas, (g) produce value-added co-products or by-products (e.g., biopolymers, proteins, polysaccharides, pigments, animal feed, fertilizer and H2), and (h) grow in enclosed culture vessels (photobioreactors) throughout the year with a potential annual biomass productivity rate 10-fold greater that of terrestrial plants.
While high oil prices, diminishing world oil reserves, and the environmental deterioration associated with fossil fuel consumption have generated renewed interest in using algae as an alternative and renewable feedstock, the path forward for algal feedstock-based biofuels from both the opportunity and challenge perspectives must be addressed. Issues dictated by cost will include, (a) the choice of optimal algal strains (many companies and the US Air Force Office of Scientific Research are sponsoring discovery programs in natural environments) suitable for the location of the production facility and the time of the year, (b) the use of open ponds versus closed bioreactors (e.g., engineering breakthroughs related to algal mass culture will have to be made), (c) the simultaneous optimization of both algal growth rates and oil production per unit time, (d) efficient cell harvesting and oil extraction methods, (e) and critical, downstream processing (biodiesel produced chemically by transesterification, using a catalyst such as sodium methylate and methanol, versus green diesel produced thermochemically by hydrogenation processes as in an oil refinery). All of these will have to be addressed before this technology can become a commercial reality. Furthermore, many fundamental biological questions related to the biosynthesis and regulation of fatty acids and TAGs in algae need to be answered. Clearly, physiological and genetic manipulations of growth and lipid metabolism must become readily implementable, as well as perhaps the successful application of modern systems biology and synthetic biology approaches.
Based upon photosynthetic efficiency and the growth potential of algae, theoretical calculations indicate that the annual production of 5,000-6,000 gallons of algal oil per acre of land might be achievable by mass culture of oleaginous algae (estimates of 100,000 gallons or more in the popular press are not realistic); this is an oil yield 100-fold greater than that of soybeans. At this point many algae-based, biofuel start-up companies (perhaps as many as 180 world wide) are trying to push oil-production technology beyond the small laboratory or field testing stage (Figure 12). However, lipid yields, obtained from algal mass culture efforts to date, fall short (at least 10 to 20 times less) of the theoretical maximum, and this has made algal oil production technology prohibitively expensive at least historically. Nevertheless, there are companies claiming current costs of photosynthetically-driven biodiesel production in the $38 per gallon range, the ability to grow algae in the US year round using cold-adapted organisms and waste heat input, and future projected costs of algal oil in the $1.50 per gallon range. Furthermore, recent success in open-pond culturing of algae year-round in northern Nevada has been verified by a university group. Finally on January 7, 2009, Continental Airlines announced the first test flight of a commercial aircraft powered by jet fuel consisting in part of an algal biofuel produced by a California-based company.

Figure 12. An aerial view of a pilot plant (Seambiotic Ltd., Tel Aviv) growing Nannochloropsis (the algae are rich in oil) using flue gas CO2 from a coal-fired Power Plant near Ashkelon, Isreal. The different sized raceway ponds are used to scale up the algae culture for inoculating the largest raceway. (thanks to Professor Ami Ben-Amotz for the photograph)
Other Products from Algae. Ethanol is another fuel that can be produced directly by algae. This technology is being licensed in Mexico, and perhaps other countries, for a scale up of large area production facilities. It is quite possible that algae might be harnessed to produce fuels other than H2, biodiesel, and ethanol as well as many other commodity and specialty products (see below for examples of current commercial products). Various researchers have estimated that between 200,000 and several million unique species of microalgae exist in the wild, and no one knows the diversity the of bioproducts that might be discovered, if there were a concerted world-wide effort to look. Chlamydomonas reinhardtii (a green alga) and many other algae produce starch, ethanol, acetate, formate and glycerol, which can be used as fuels or substrates for other organisms that can produce fuels. It also expresses the gene for butyrate, which might be turned into butanol either by adding genes to complete the pathway in the organism, or by chemically converting the butyrate to butanol. Furthermore, the production of many of the products of various fermentations and other metabolisms discussed above for first- and second-generation technologies, including higher alcohols, 1,3-propanediol, polyethylene, amino acids, and polylactic acid, might also be engineered into algae at some time in the future. There have also been suggestions for using algae to produce useful enzymes.
At this point there is a sizable market for microalgal biomass (several billion $US worldwide in 2004). Various species are used in the human health market (e.g., Chlorella, Dunaliella, and Spirulina); as animal feed additives, as a food source in the aquaculture industry, as sources of coloring agents (astaxanthin, phycocyanin, phycoerythrin, lutein, zeaxanthin, and canthaxantin) in, e.g., salmon and chicken production, as sources of antioxidants or anti-inflammatory products (β-carotene, tocopherol [vitamin E], omega-3 fatty acids [polyunsaturated fatty acids or PUFAs including alpha-linolenic acid or ALA, eicosapentaenoic acid or EPA, and docosahexaenoic acid or DHA]); for the production of specialty toxins; and for the generation of stable isotope-labeled biochemicals. Other recent developments include the use of algae to synthesize biochemicals for pharmaceutical applications. Some additional examples under consideration are the production of anti-HIV and anti-fungal agents, treatments for carpal tunnel syndrome, treatments for irritable bowel candidiasis, and newer types of antioxidant and anti-inflammatory agents.
Finally, there are also a number of projects around the world that are examining the potential of feeding algae with waste CO2 as a way of recycling the gas for fuels production, environmental protection applications, and global climate change amelioration.
Suggested Reading
Bridgwater, A, S. Czernik, J. Diebold, D. Meier, A. Oasmaa, C. Peacocke, J. Piskorz, D. Radlein (1999) Fast Pyrolysis of Biomass: A Handbook. CPL Press, Tall Gables, UK, pp. 180. (Covers many of the topical areas discussed in this Chapter in greater detail.)
Brown, R.C. (2003) Biorenewable Resources: Engineering New Products from Agriculture. John Wiley & Sons, Limited, Hoboken, NJ, pp. 286. (Covers many of the topical areas discussed in this Chapter in greater detail.)
Chisti, Y. (2007) "Biodiesel from Microalgae", Biotechnology Advances 25, 294-306. (Oriented toward growth and bioreactor information.)
Decker, S.R., J. Sheehan, D.C., Dayton, J.J Bozell, W.S. Adney, B. Hames, S.R Thomas, et al. (2007) "Biomass Conversion", in Kent and Riegel's Handbook of Industrial Chemistry and Biotechnology (Kent, J.A., Ed.), Springer Science, New York. Eleventh Edition, Vol. 2, Chapter 33, pp. 1499-1548. (Covers many areas of plant biomass treatment.)
Ghirardi M.L., M. Posewitz, Maness P-C., Dubini, A., Yu J., and M. Seibert (2007) "Hydrogenases and Hydrogen Photoproduction in Oxygenic Photosynthetic Organisms", Ann. Rev. Plant Biol. 58, 71-91. (A recent review of the algal biohydrogen area.)
Himmel, M.E., Ed. (2007) Biomass Recalcitrance. Blackwell Publishing, West Sussex, U.K. (Covers many areas of plant biomass treatment.)
Huber, G.W., S. Iborra, and A. Corma. (2006) Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering Chem. Rev. 106, 4044-4098. (Covers many of the topical areas discussed in this Chapter in greater detail.)
Hu, Q., M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz., M. Seibert, and A. Darzins (2008) "Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances", The Plant J. 54(4), 621-639. (A recent review of the algal biodiesel area.)
Kosourov, S.N. and M. Seibert (2009; published Online July 2008) "Hydrogen Photoproduction by Nutrient-Deprived Chlamydomonas reinhardtii Cells Immobilized within Thin Alginate Films under Aerobic and Anaerobic Conditions," Biotechnology and Bioengineering, 102 (2), 50-58. (An immobilized wild-type algal system reporting the highest yet light to hydrogen energy conversion efficiency.)
Pulz, O., and W. Gross (2004) "Valuable Products from Biotechnology of Microalgae", Appl. Microbiol. Biotechnol. 65, 635-648. (Covers algal products other than fuels.)
Seibert, M., P. King, M.C. Posewitz, A. Melis, and M.L. Ghirardi (2008) "Photosynthetic Water-Splitting for Hydrogen Production," in Bioenergy (J. Wall, C. Harwood, and A. Demain, Eds.) ASM Press, Washington DC, pp. 273-291. (A recent review of the algal biohydrogen area including hydrogenases and omic studies.)
Sheehan, J., Dunahay, T., Benemann, J., and Roessler, P.G. (1998). U.S. Department of Energy's Office of Fuels Development, July 1998. A Look Back at the U.S. Department of Energy's Aquatic Species Program--Biodiesel from Algae. Close Out Report, National Renewable Energy Laboratory/TP-580-24190, pp. 294. (Summarizes the results of the US Department of Energy's Aquatic Species Program.)
Wall, J., C. Harwood, and A. Demain, Eds. (2008) Bioenergy. ASM Press, Washington DC. pp. 437. (Covers many of the topical areas discussed in this Chapter in greater detail.)
Weaver, P. F., S. Lien, and M. Seibert (l980) "Photobiological Hydrogen Production", Solar Energy 24, 3-45. (Covers the older literature related to biohydrogen related research.)
03/02/09
03/26/09