PHOTOBIOLOGY of the HUMAN LENS
Joan E. Roberts
Fordham University, Department of Natural Sciences
113 West 60th Street, New York City, NY 10023
jroberts@fordham.edu
Introduction
The primary function of the human lens is to focus light undistorted onto the retina. While the transmission properties of most of the components of the eye are stable, the transmission properties of the lens change throughout life, as seen in Figure 1.
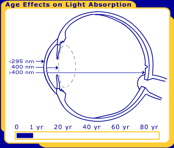
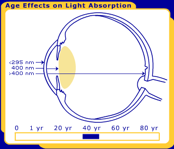
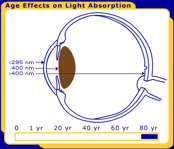
Figure 1. The changes in the human lens throughout life. Pictured are at birth, 40 years and 80 years.
Exposure to the intense light of the sun can pose a particular hazard to the lens of the eye, and lead to the formation of a cataract [a clouding of the lens], which impairs vision. Both UV-A and UV-B exposure [Andley et al. 2004; Roberts 2001; Balasubramanian 2000] are major risk factors for the induction of a cataract, especially in those above 70 years old, because with age the eye's ability to protect itself against light damage is compromised. Exposure to UV radiation from the reflection off of water, sand, or snow is particularly damaging to the lens of the eye [Sliney 2005; Merriam 1996; Coroneo 1990]. In addition to UV radiation alone, there are many dyes, drugs and herbal medication that in the presence of both visible light and UV radiation can induce a cataract [Roberts 2002]. This phototoxic reaction causes a very early cataract [about 40 years old].
Any modification in the clarity of the lens will degrade the quality of the image presented to the retina, and greatly affects visual perception. In this module, we will learn about the photochemistry and photobiology of the lens, and how these properties affect not only the retina, but overall human health [Roberts 2000].
Structure of the Front of the Eye (Anterior Segment)
The human eye is composed of several compartments, as seen in Figure 2. The outermost layer contains the sclera, whose function is to protect the eyeball, and the cornea, which focuses incoming light onto the lens. Beneath this layer is the choroid containing the iris, which is known as the uvea. This region contains melanocytes, which contain the pigment melanin, whose function is to prevent light scattering. The opening in the iris, the pupil, expands and contracts to control the amount of incoming light. The iris and the lens are bathed in the aqueous humor. The aqueous humor is a fluid that serves as a transparent circulatory system (what blood flow does in non-transparent tissues). It not only maintains intraocular pressure, but also provides nutrition to the lens and cornea, and removes debris and waste from these ocular tissues. The aqueous humor contains high concentrations of various antioxidants. The lens is positioned behind the iris. The function of the lens is to focus light undistorted onto the retina, which is in the back of the eye (posterior segment) [Roberts 2001].

Figure 2. The structure of the Human Eye.
The Structure of the Human Lens
The structure of the human lens is seen in Figure 3. The lens is a transparent organ located behind the cornea and the iris [Bachem 1956]. The outer edge of the lens consists of a single layer of epithelial cells, and a membrane that covers the entire organ [Kuszak 1994]. Lens epithelial cells do not divide except when undergoing repair. Some epithelial cells lose their nuclei and other organelles, and become lens fiber cells [Bassnett and Mataic 1997]. These lens fiber cells are filled with a 30% solution of protein, known as cytosol (soluble) lens protein. Because there is little protein turnover in the lens fiber cells, damage to lens protein accumulates throughout life.

Figure 3. The structure of the Human Lens.
Suture and Equator are anatomical terms in ophthalmology. Suture means the seams of the lens. The suture patterns become more complex as more layers of lens fibers are added to the outer portion of the lens. Equator means the edge of the largest portion of the lens (similar to the equator on a globe).
When is Light Harmful to the Human Lens?
Although environmental light is mostly benign, there are several conditions under which environmental light exposure becomes harmful. To determine whether light is damaging, one must consider the following factors: intensity, wavelength, site of damage, oxygen tension, chromophores, defense systems, and repair mechanisms.
Intensity. The greater the intensity of light, the more likely it is to damage the eye. Light that may not ordinarily be harmful can do acute damage if it is sufficiently intense. For example, it is well known that the eye can be damaged (temporarily or permanently) by exposure to reflective sunlight from snow (snow blindness), or from staring at the sun during an eclipse [Sliney 2005]. There is an increase in UV radiation with a thinning of the protective ozone layer [Norval 2007]. Similarly, the eye can sustain damage from artificial light sources that emit UV-A or UV-B [Sliney 1997]. Cumulative light damage results from less intense exposure over a longer period of time, and is often a result of an underlying age related loss of protection [Giblin 2000; Seth and Kharb 1999; Yeum 1999].
Wavelength. Ambient radiation, from the sun or from artificial light sources, contains varying amounts of UV-C (100-280 nm), UV-B (280-315 nm), UV-A (315-400 nm), and visible (400-700 nm) light. The shorter the wavelength, the greater the energy, and therefore the greater the potential for biological damage. However, although the longer wavelengths are less energetic, they penetrate the eye more deeply [Roberts 2001].
In order for a photochemical reaction to occur, the light must be absorbed in a particular ocular tissue. The primate/human eye has unique filtering characteristics that determine in which area of the eye each wavelength of light will be absorbed. UV radiation below 295 nm is filtered from reaching the lens by the human cornea. This means that the shortest, most energetic wavelengths of light (all UV-C and some UV-B) are filtered out before they reach the human lens. Most UV light is absorbed by the lens, but the exact wavelength range depends upon age. In adults, the lens absorbs the remaining UV-B and all of UV-A (295-400 nm), and therefore only visible light reaches the retina. However, the very young human lens transmits a small window of UV-B light (320 nm) to the retina, while the elderly lens filters out much of the short blue visible light (400-500 nm). Transmission also differs with species; the lenses of mammals other than primates transmit UV radiation longer than 295 nm to the retina [Barker et al. 1991; Bachem 1956].
Site of Light Damage to the Lens. The lens is composed of two parts that are most susceptible to damage: the (outer) epithelial cells and the (inner) fiber membrane. The epithelial cells control transport to the lens. They have direct contact with the aqueous humour, and are most vulnerable to phototoxic damage. Damage to these cells would readily compromise the viability of the lens [Andley et al. 1994]. The fiber membrane can be photochemically damaged through damage to the lipids and/or to the main intrinsic membrane protein [Schey et al. 2000; Roberts et al. 1985].
Phototoxic reactions can lead to a modification of DNA and certain amino acids (histidine, tryptophan, cysteine) and/or a covalent attachment of the sensitizer to cytosol lens proteins [Roberts 2002]. Covalently bound chromophores may then act as endogenous sensitizers, and produce prolonged sensitivity to light. In addition, there is non-photochemically induced modification of lens proteins associated with diabetes [Argirov 2004; Argirova and Breipohl 2002]. A high glucose concentration has been found to lead to the glycosylation of epsilon-amino groups of lysine residues. All of these types of damage will result in a change in the refractive index of the lens material, leading to aggregation and ultimately opacification (cataractogenesis) [Benedek, G. B. (1971]. A recently developed technique (ScanTox) measures very early changes in the optical quality (focusing) of the lens, even before damage causes opacification of the lens [ Dovrat and Sivak 2005].
Chromophores. A chromophore is a substance that absorbs light. An ocular chromophore can be either an endogenous compound naturally present in the eye, or an exogenous agent that has passed through blood-ocular barriers and penetrated to a particular site. In order for light to damage the lens, the light must first be absorbed by a chromophore located in some compartment of the lens.
a) Endogenous (Naturally Occurring) Chromophores in the Human Lens. The chromophores in the human lens change throughout life as seen in Figure 4a and b. There is actually little damage to the human eye from light before middle age. This is because the adult human lens contains yellow chromophores (3-hydroxykyurenines) that absorb light, but release the energy before it has a chance to do any damage [Dillon and Atherton 1990]. So the kynurenine chromophores present in the adult human lens are not only safe, but serve to protect the retina by filtering UV radiation, thus preventing it from reaching and damaging the retina [Dillon 1991]. After middle age an enzyme (kynurenine amino transferase), produced in increasing amounts, converts the protective chromophores (3-OH kynurenine and its glucoside) into destructive chromophores, xanthurenic acid [Malina and Martin 1996] and xanthurenic glucoside [Balasubramanian 2005]. When these xanthurenic compounds absorb light, they produce reactive oxygen species (singlet oxygen and/or superoxide) [Roberts et al. 2000; Thiagarajan et al. 2002], which damage lens proteins [Roberts et al. 2001]. Another chromophore, N-formyl kynurenine, formed from the continual photooxidation of endogenous tryptophan [Finley et al. 1998], also produces singlet oxygen and superoxide, which damage lens proteins [Krishna et al. 1991]. Thus, xanthurenic acid and N-formyl kynurenine are likely candidates for the chromophores responsible for age-related cataract formation.

Figure 4a. Age Related Changes in the Human Lens. As the lens ages, it's chromophores change the color of the human eye from clear (middle) to yellow (top right). As a result of aging changes in the human lens a clouding of the lens occurs, which is known as a cataract (top left). Cow lenses (bottom) and other non-primates have clear lenses throughout life.
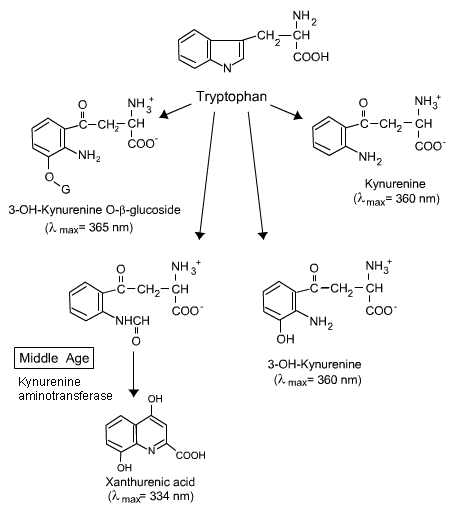
Figure 4b. The change in tryptophan derivatives in the human lens with age. Note the change at Middle Age. For more information, see the text above.
b) Xenobiotics or Exogenous [External] Chromophores in the Lens. Intense or accumulated UV-B or UV-A radiation causes direct damage to the human lens. However, in the presence of a light activated (photosensitized) drug, herbal medication, (hypericin in St. Johns Wort) or nanoparticles, patients are in danger of enhanced ocular injury from ambient UV radiation and visible light [Roberts 2002, 2008; Roberts et al. 2008]. The extent to which a particular chemical is capable of producing phototoxic side effects in the eye depends on several parameters including: 1) the chemical structure; 2) the absorption spectra of the drug; 3) binding of the drug to ocular tissue; and 4) the ability to cross blood-ocular barriers.
Any compound that has a tricyclic, heterocyclic or porphyrin ring structure is a potential ocular chromophore if it has absorbance above the cut off of the cornea (>295 nm). When these exogenous (external) sensitizers bind to ocular tissues (i.e., lens proteins), their retention time in the lens is extended, and the potential hazard they pose is enhanced. Substances that are amphiphilic or lipophilic [i.e., soluble in either water or lipid] are able to cross most lenticular barriers [Roberts 2002]. The lens is fed by the aqueous humour, and it is relatively difficult for a substance to pass through the aqueous humour to the lens by ingestion. However, once in the lens, it is also difficult for the foreign substance to be removed.
Oxygen Tension. The oxygen tension in the lens is very low, but is sufficient for photooxidation to occur [McLaren et al. 1999; Kwan et al. 1971].
Defense Systems. The lens has a very efficient defense system against light and radiation damage. The lens contains antioxidant enzymes (superoxide dismutase (SOD) and catalase), and antioxidants (Vitamin E, C, lutein, glutathione) that serve to protect it against oxidative and photoinduced damage [ Roberts 2001; Khachik 1997; Balasubramanian 2005]. Unfortunately, most of these antioxidants and protective enzymes decrease beginning at forty years of age [Samiec et al. 1998; Lyle et al. 1999], leaving the lens defenseless against light damage.
Repair. The peripheral lens epithelial cells are able to repair UV-B induced DNA cross-links (cyclobutane pyrimidine dimers and 6-4 pyrimidine-pyrimidone) [Andley et al. 1999], but any additional exposure to UV-A [Zigman et al. 2000; Ayala et al. 2000] interferes with cell repair. As there is little turnover of lens proteins, damage to lens proteins acumulates [Roberts 2002].
Mechanism of Light Damage to the Lens
Photooxidation. Intense light can induce direct DNA damage, but with less intense light, the eye is damaged through a phototoxidation reaction. In photooxidation reactions, a chromophore in the eye absorbs light and oxidizes certain amino acids and/or nucleic acids, which results in damage to the whole lens. The chromophore may be endogenous (natural) or exogenous (drug, herbal medication or nanoparticle that has accumulated in the eye). The absorption of light excites the chromophore to an excited singlet state, which then undergoes intersystem crossing, and reaches the triplet state. In its triplet state, the chromophore then proceeds either via a Type I (free radical) or Type II (singlet oxygen) mechanism to cause the eventual damage [Straight and Spikes 1985]. Photooxidation can occur in the lens by either a Type I or a Type II mechanism, or both concurrently.
The chromophores is the adult human lens may be excited by light, but they come down from this excited state (singlet) very quickly (nanoseconds), so they don't have the chance of reaching a triplet state, of making damaging active intermediates, and therefore of causing damage in the lens [Dillon and Atherton 1990; Dillon 1991]. However, when the efficient photosensitizers, xanthurenic acid, it's glucoside and N-formyl kynurenine are present in the lens and the lens is exposed to UV radiation, they are capable are making triplets with sufficient efficiency (quantum yield) to form reactive oxygen species and free radicals, which then in turn damage lens tissue[Balasubramanian 2005; Roberts 2000; Thiagarajan 2002].
Cataracts
Mechanism of Induction. The human lens is normally transparent until the age of 40 years. This transparency is a result of the orderly arrangement of protein fibers in the lens normally [Benedek 1971]. At middle age, the eye's natural enzymatic and antioxidant protection against UV-A and UV-B is lost at the same time there is an increase in the production of photochemically active chromophores. As the lens absorbs ambient light, these chromophores are photoactivated and produce reactive oxygen species, such as singlet oxygen and superoxide. The lens proteins (alpha, beta, gamma crystallins) become denatured, or the lens epithelial cells can no longer repair damage from ambient light [Roberts et al. 2001; Finley et al. 1998; Krishna et al. 1991; Andley 2007]. By the age of 70, the lens finally becomes sufficiently cloudy to obstruct vision, and the individual is said to have an age-related cataract (Figure 4a) [Roberts 2002].
Cataracts can also develop at a much earlier age [40 years old] when the person is exposed to excessive UV radiation, cigarette smoke and air pollution, photosensitizing medication, steroids or has diabetes. The underlying cause of these cataracts is also oxidative (and phototoxidative) damage to the lens epithelial cells and lens proteins.
Maintenance of structural integrity is particularly important for lens protein alpha-crystallin because of its role as a molecular chaperone. alpha-Crystallin is an aggregate of two polypeptides, A and B, which are small heat shock proteins that prevent UV (A and B)-induced protein aggregation [Andley 2008, 2007]. By adding and removing alpha-crystallin production from lens epithelial cells, Andley has shown that alpha-crystallin confers natural protection against UV radiation damage to lens cells [Andley 2007]. alpha-Crystallin also protects against the UV-A inhibition of protective (catalase) enzyme activity [Horwitz and Zigman 1997]. The specific sites of damage to alpha-crystallin with both endogenous and exogenous chromophores have been detected using mass spectrometry [Roberts et al. 2001; Finley et al. 1997,1998a,b; Schey 2000] and monoclonal antibody techniques [Staniszewska and Nagaraj 2005]. Advanced Glycation End-products [AGE] found in diabetic cataracts may also behave as photosensitizers and oxidize lens proteins [Argirov et al. 2004; Argirova and Breipohl 2002].
All endogenous or exogenous oxidation denatures the lens proteins, reduces their solubility, and eventually, results in a loss of transparency in the lens, which is known as a cataract. A cataract that occurs in the central portion of the lens is known as a nuclear cataract, and those that occur in the periphery of the lens are known as cortical cataracts. A rarer form of cataract is known as a posterior subcapsular cataracts. This cataract is generally thought to be genetically linked, and occurs at birth or very early age, or as a result of steroid use or diabetes [Bochow 1989].
Diagnosis and Treatment. Cataracts may be easily diagnosed with the use of a "slit lamp" or an ophthalmoscope, which examines the lens for lack of transparency, and determines the location and the density of the clouding. In addition, a visual acuity test [the eye chart with progressively smaller letters from top to bottom] will determine how well the patient can see with the cataract. When significant loss of vision is noted, the treatment is to surgically remove the lens. This lens is commonly replaced with an intraocular plastic lens containing a UV-A and UV-B filter, to replace the focusing and filtering power lost from the cataract lens removal [van Norren and van de Kraats 2007; Sliney 2007]. Recently, intraocular artificial lens have been available with short blue light filters (400 - 440 nm), which are important to protect the elderly from macular degeneration and the diabetic from diabetic retinopathy [Rodriguez-Galietero et al. 2005; Falkner-Radler et al. 2008].
Prevention. If you prevent light from exciting endogenous or exogenous chromophores in the lens, or you block the damage of reactive oxygen species with antioxidants, you may prevent or retard cataracts from forming [Roberts 2008].
a) Sunglasses. Both UV-A and UV-B are not necessary for either sight or to trigger the circadian response. On the other hand, both UV-A and UV-B induce cataract formation. The removal of these wavelengths from ocular exposure will greatly reduce the risk of early cataract formation. This may be easily done by wearing sunglasses that block wavelengths below 400 nm [marked 400 on the glasses]. However, because of the geometry of the eye [Sliney 2005; Merriam 1996] these glasses must be wraparound sunglasses to prevent reflective UV radiation from reaching the eye.
b) Antioxidants Since age decreases the normal production of antioxidants in the lens [Khachik et al. 1997; Balasubramanian 2005; Samiec et al. 1998; Lyle et al. 1999; Busch et al. 1999], increasing the eating fruits and vegetables has been suggested to replace the missing protection [Jacques et al. 2001; Lyle et al. 1999]. In addition, supplementation with vitamins and antioxidants, including vitamin E and lutein, have been shown to be particularly effective in retarding age related cataracts [Schalch and Chylack 2003; Olmedilla 2003; Edge et al. 1997].
Supplements should be balanced, because damaging oxidation reactions can occur if only one antioxidant is taken [Edge et al. 1998]. In the AREDS (Age-Related Eye Disease Study) sponsored by the National Eye Institute, it was found that excessive beta-carotene was linked with an increased risk for lung cancer for smokers, while excessive Zn was linked with an increased risk of prostate cancer. As lutein, not beta-carotene, is the natural carotenoid found in the lens and retina [Khachik et al. 1997], supplementation with excessive beta-carotene is not only unnecessary to protect the eye, but is hazardous to smokers and former smokers. Other natural products such as green tea, which contains polyphenols (epigallocatechin gallate) [Zigman et al. 1999; Zigman 2000] and Ashwagandha (root of Withania somnifera) used in traditional Ayurvedic medicine has also been shown to retard light induced damage to the lens [Balasubramanian 2005; Thiagarajan et al. 2003].
Conclusions
Cataract formation is an age related disease. Most people will form a cataract by the time they are 70 years old. Both UV-A and UV-B are very important risk factors for the development of early cataracts. In addition, exposure to even visible light in the presence of steroids, photosensitizing drugs, cosmetics and nanoparticles may dramatically increase the risk of early cataracts. UV radiation avoidance with appropriate sunglasses, and the appropriate combination of oxidizing and reducing antioxidants [lutein, zeaxanthin, vitamin E, C, Zn and Cu] supplementation may help retard or eliminate this blinding disorder in the elderly.
References
Andley UP (2008) The lens epithelium: focus on the expression and function of the alpha-crystallin chaperones. Int J Biochem Cell Biol. 40:317-23.
Andley UP (2007) Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 26:78-98.
Andley U P, Rhim JS , Chylack Jr LT, Fleming TP (1994) Propagation and immortalization of human lens epithelial cells, Invest. Ophthalmol. Vis. Sci., 35:3094-3102.
Andley UP, Patel HC, Xi JH, Bai F (2004) Identification of genes responsive to UV-A radiation in human lens epithelial cells using cDNA microarrays. Photochem. Photobiol. 80, 61-71.
Andley UP, Song Z, Mitchell DL (1999) DNA repair and survival in human lens epithelial cells with extended lifespan. Curr Eye Res.18:224-30.
Argirov OK, Lin B, Ortwerth BJ (2004) 2-ammonion-6- (3-oxidopyridinium-1-yl) hexanoate (OP-lysine) is a newly identified advanced glycation end product in cataractous and aged human lenses. J. Biol. Chem. 279:6487-6495.
Argirova MD, Breipohl W (2002) Glycated proteins can enhance photooxidative stress in aged and diabetic lenses. Free Radic. Res. 36:1251-1259.
Ayala MN, Michael R, Soderberg PG (2000) Influence of exposure time for UV radiation-induced cataract Invest Ophthalmol Vis Sci. 41: 3539-43. Bachem, A. (1956) Ophthalmic action spectra. Am. J. Ophthalmol. 41: 969-975.
Balasubramanian D (2000) Ultraviolet radiation and cataract. J. Ocular Pharmacol. Therap. 16, 285-297.
Balasubramanian D (2005) Photodynamics of Cataract: An Update on Endogenous Chromophores and Antioxidants. Photochem. Photobiol. 81:498-501.
Benedek GB (1971) Theory of transparency of the eye. Appl. Optics 10:459-473.
Bochow TW, West SK, Azar A, Munoz B, Sommer A, Taylor H R (1989) Ultraviolet light exposure and risk of posterior subcapsular cataracts Arch. Ophthalmology 107: 369-372.
Barker, FM, Brainard GC and Dayhaw-Barker P (1991) Transmittance of the human lens as a function of age. Invest. Ophthalmol. Vis. Sci. 32S p. 1083.
Bassnett S and Mataic D (1997) Chromatin Degradation in Differentiating Fiber Cells of the Eye Lens J. Cell Biol. 137: 37-49.
Busch M, Gorgels TG, Roberts JE, van Norren D (1999) The effects of two stereoisomers of N-acetylcysteine on photochemical damage by UVA and blue light in rat retina. Photochem. Photobiol. 70:353-358.
Coroneo MT (1990) Albedo Concentration in the Anterior Eye: a Phenomenon that Locates Some Solar Diseases. Ophthalmic. Surg. 21: 6066.
Dillon J and Atherton SJ (1990) Time Resolved Spectroscopic Studies on the Intact Human Lens. Photochem. Photobiol. 51 :465-468.
Dillon J (199) Photophysics and Photobiology of the Eye. J. Photochem. Photobiol. B BioI. 10:23-40.
Dovrat A, Sivak JG (2005) Long-term lens organ culture system with a method for monitoring lens optical quality. Photochem Photobiol. 81:502-505.
Edge R, Land EJ, McGarvey DJ, Mulroy L, Truscott TG (1998) Relative one-electron reduction potentials of carotenoid radical cations and the interactions of carotenoids with the vitamin E radical cation. J. Am. Chem. Soc. 120:4087-4090.
Edge R, McGarvey DJ, Truscott TG (1997) The carotenoids as anti-oxidants - a review. J. Photochem. Photobiol. B: Biol. 41:189-200.
Falkner-Radler CI, Benesch T, Binder S. (2008) Blue light-filter intraocular lenses in vitrectomy combined with cataract surgery: results of a randomized controlled clinical trial.Am J Ophthalmol. 145:499-503.
Finley EL, Dillon J, Crouch RK, Schey KL (1998). Identification of Tryptophan Products in Oxidation Bovine Alpha-Crystallin. Protein Sci., 7:2391-2397.
Finley EL, Dillon J, Crouch RK, Schey KL (1998) Radiolysis-induced oxidation of bovine alpha-crystallin. Photochem. Photobiol. 68:9-15.
Finley EL, Busman M, Dillon J, Crouch RK, Schey KL (1997) Identification of photooxidation sites in bovine alpha-crystallin. Photochem. Photobiol. 66:635-641.
Giblin FJ (2000) Glutathione: a Vital Lens Antioxidant. J. Ocul. Pharmacol. Ther. 16: 121-135.
Horwitz J, Zigman S (1997) Do alpha-crystallins protect catalase against UV damage? Biol Bull. 193:254-255.
Jacques PF, Chylack LT Jr, Hankinson SE, Khu PM, Rogers G, Friend J, Tung W, Wolfe JK, Padhye N, Willett WC, Taylor A. (2001) Long-term nutrient intake and early age-related nuclear lens opacities. Arch Ophthalmol. 119:1009-1019.
Khachik F, Bernstein PS, Garland DL (1997) Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest. Ophthalmol. Vis. Sci. 38 pp. 1802-1811.
Krishna CM, Uppuluri S, Riesz P, Zigler Jr JS, Balasubramanian D (1991) A Study of the Photodynamic Efficiencies of Some Eye Lens Constituents. Photochem. Photobiol. 54:51-58.
Kuszak JR, Peterson KL, Sivak JG, Herbert KL. (1994) The interrelationship of lens anatomy and optical quality. II. Primate lenses. Exp Eye Res. 59:521-35.
Kwan M, Niinikoske J, Hunt TK (1971) Oxygen tension in the aqueous and the lens. Invest. Ophthalmol. 11:108-111.
Lyle BJ, Mares-Perlman JA, Klein BE, Klein R, Greger JL (1999) Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am. J. Epidemiol. 149:801-809.
Malina HZ, Martin XD (1996) Xanthurenic Acid Derivative Formation in the Lens is Responsible for Senile Cataract in Humans. Graefes Arch. Clin. Exp.Ophth. 234:723-730
McLaren JW, Dinslage S, Dillon JP, Roberts JE, Brubaker RF (1999) Measuring oxygen tension in the anterior chamber of rabbits. Invest. Ophthalmol. Vis. Sci. 39:1899-1909.
Merriam JC (1996) The Concentration of Light in the Human Lens. Trans. Am. Ophthalmol. Soc. 94: 803-918.
Norval M, Cullen AP, de Gruijl FR, Longstreth J, Takizawa Y, Lucas RM, Noonan FP, van der Leun JC. (2007). The effects on human health from stratospheric ozone depletion and its interactions with climate change. Photochem Photobiol Sci. 6:232-51.
Olmedilla B, Granado, F, Blanco I, Vaquero M (2003) Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-year double blind, placebo-controlled pilot study. Nutrition 19, 21-24.
Roberts JE (2008) "Drug Induced Ocular Phototoxicity" In: Marzulli and Maibach's Dermatotoxicology, 7th Edition, edited by H. Zhai, K-P Wilhelm, and H. Maibach, Chapter 28, pp 269-278. Taylor & Francis Group, Boca Raton, Florida.
Roberts JE, Wielgus AR, Boyes WK, Andley U, Chignell CF. (2008) Phototoxicity and cytotoxicity of fullerol in human lens epithelial cells. Toxicology and Applied Pharm 228:49-58.
Roberts JE (2002) Screening for Ocular Phototoxicity. International Journal of Toxicology 21:491-500.
Roberts JE, Finley EL, Patat SA, Schey K L (2001) Photooxidation of Lens Proteins with Xanthurenic Acid: A Putative Chromophore for Cataractogenesis. Photochem. Photobiol. 74: 740-744.
Roberts JE. (2001) Ocular phototoxicity. J. Photochem. Photobiol. B: Biology 64, 136-143.
Roberts JE, Wishart JF, Martinez L Chignell CF (2000) Photochemical Studies on Xanthurenic Acid. Photochem. Photobiol. 72: 467471.
Roberts JE (2000) Light and Immunomodulation. NY Acad Sci. 917:435-445.
Roberts JE, Roy D, Dillon J (1985) The photosensitized oxidation of the calf lens main intrinsic protein (MP26) with hematoporphyrin. Curr. Eye Res. 4:l8l-185.
Rodriguez-Galietero A, Montes-Mico R, Munoz G, Albarran-Diego C. (2005) Blue-light filtering intraocular lens in patients with diabetes: contrast sensitivity and chromatic discrimination. J Cataract Refract Surg. 31:2088-2092.
Samiec PS, Drews-Botsch C, Flagge EW, Kurtz JC, Sternberg P, Reed RL Jones DP (1998) Glutathione in human plasma declines in association with aging, age-related macular degeneration and diabetes. Free Radic. Biol. Med. 24:699-704.
Schalch W and Chylack LT Jr, (2003) Antioxidant micronutrients and cataract. review and comparison of the AREDS and REACT cataract studies. Ophthalmologe 100, 181-189.
Schey KL, Little M, Fowler JG, Crouch RK (2000) Characterization of human lens major intrinsic protein structure, Invest. Ophthal. Vis Sci., 41 175-182.
Schey KL, Patat S, Chignell CF, Datillo M, Wang RH, Roberts JE (2000) Photooxidation of lens proteins by hypericin (active ingredient in St. John's Wort). Photochem. Photobiol. 72:200-207.
Seth RK, Kharb S (1999) Protective Function of Alpha-tocopherol Against the Process of Cataractogenesis in Humans. Ann. Nutr. Metab. 43: 286-289.
Sliney DH (2007) Comment : Spectral transmission of IOLs expressed as a virtual age. Br J Ophthalmol. 91:1261-1262.
Sliney DH (2005) Exposure geometry and spectral environment determine photobiological effects on the human eye. Photochem Photobiol. 81:483-489.
Sliney, D.H. (1997) Optical radiation safety of medical light sources. Phys. Med. Biol. 42:981-996.
Straight R, Spikes JD (1985) Photosensitized oxidation of biomolecules. In: O. Singlet, Editor, A.A. Frimer, Editor, Polymers and Biopolymers Vol. IV, CRC Press, Boca Raton, FL, pp. 91-143.
Staniszewska MM, Nagaraj RH. (2005) 3-hydroxykynurenine-mediated modification of human lens proteins: structure determination of a major modification using a monoclonal antibody. J Biol Chem. 280:22154-64.
Thiagarajan G, Venu T, Balasubramanian D (2003) Approaches to relieve the burden of cataract blindness through natural antioxidants: use of Ashwagandha (Withania somnifera). Curr. Sci. 85, 1065-1071.
Thiagarajan G, Shirao E, Ando K, Inoue A, Balasubramanian D (2002) Role of xanthurenic acid 8-O-beta-glucoside, a novel fluorophore that accumulates in the brunescent human eye lens. Photochem. Photobiol. 76, 368-372.
van Norren D, van de Kraats J. (2007) Spectral transmission of intraocular lenses expressed as a virtual age. Br J Ophthalmol; 91: 1374-1375.
Yeum KJ, Shang FM, Schalch WM, Russell RM, Taylor A (1999) Fat-soluble nutrient concentrations in different layers of human cataractous lens. Curr.Eye Res. 19: 502-505.
Zigman S, McDaniel T, Schultz J, Reddan J (2000) Effects of intermittent UVA exposure on cultured lens epithelial cells. Curr Eye Res. 20:95-100.
Zigman S, Rafferty NS, Rafferty KA, Lewis N (1999) Effects of green tea polyphenolsof green tea polyphenols on lens photooxidative stress. Biol-Bull. 197: 285-286.
Zigman S (2000) Lens UVA photobiology. J Ocul Pharmacol Ther. 16:161-165.
02/25/09
09/14/11