NON-VISUAL PHOTORECEPTION in INVERTEBRATES
Carlo Musio1 and Silvia Santillo2
1CNR Istituto di Biofisica - Unità di Trento
c/o FBK, Via Sommarive 18, 38123 Trento, Italy
carlo.musio@cnr.it
2CNR Istituto di Cibernetica "Eduardo Caianiello",
Via Campi Flegrei 34, 80078 Pozzuoli (NA), ITALY
s.santillo@cib.na.cnr.it
1. Introduction
This module reviews an unconventional kind of light-sensitivity mainly present in organisms of lower animal phyla, but also recently identified in several higher vertebrate species. Particularly in invertebrates, this unconventional photosensory capability, named non-visual or non-image-forming photoreception (previously termed extraocular photoreception) does not occur in eyes or eye-like structures, or at the retinal level. Rather, it is carried out by single or clustered cells scattered throughout the whole body, or it is confined to central nervous system cells or neural pathways. In both invertebrates and vertebrates, non-visual photoreception is involved in the regulation of several expressions of temporal physiology, such as photoperiodism, locomotion, circadian rhythms, and the timing of biological clocks. It represents a parallel light-detection pathway integrating, to some extent, the function of canonical visual system, which produces image-formation, visual perception and spatial vision.
About two thirds of animal species have auxiliary extraocular, non-visual photoreceptor (NVP) systems suitable for light detecting and non-image forming functions (Land and Nilsson, 2002). In invertebrates, photic information mediated by NVP complements canonical visual activity, being involved in circadian vision (i.e., the measurement of daily environmental irradiance to serve photoentrainment of circadian rhythms), and in temporal and behavioral physiology of the animal (e.g., photoperiodism in locomotion and reproduction, timing of circadian rhythms) (Wolken, 1988; Musio, 1997; Foster and Helfrich-Förster, 2001; Guido, 2002).
Non-visual photoreception is less known and not well understood in vertebrates; however, it regulates a range of functions, including: light-mediated resetting of the biological clock, the sleep/wake cycle, melatonin inhibition, and the constriction of the pupil (Foster and Hankins, 2002, Bertolucci and Foà, 2004; Fu et al., 2005).
Until recently, cones and rods were believed to be the only cells in the retina capable of detecting light, but in 1998 a novel photopigment, called melanopsin, was discovered in the frog's skin melanophores, brain, and in retina (Provencio et al., 1998). Melanopsin is exclusively involved in non-visual photoreception. A human melanopsin has been found to be expressed in a subset of retinal ganglion cells named ipRGCs (intrinsically photosensitive retinal ganglion cells), which act as photoreceptors, but do not participate in the classic photoreceptive tasks culminating in image formation and spatial vision (Provencio et al., 2000). Without doubt, the melanopsin discovery, added to later identifications of non-visual photoreceptor cells and pigments, has radically changed our concept on vision (Foster and Hankins, 2007; Hankins et al., 2008).
In both vertebrates and invertebrates, non-visual photosensitive cells are generally located within central and peripheral regions of the nervous system, or widely diffused along dermal tissues of the animal, they share with retinal photoreceptors the same photopigment, an opsin-based protein, and the same G-coupled phototransduction scheme varying in some molecular and functional aspects (Wolken and Mogus, 1981; Musio, 1997; Foster and Hankins, 2002; Vigh et al., 2002).
These so-called "non-visual" opsins have been identified in retinal and extraretinal photosensitive neurons in both vertebrate and invertebrate species. These achievements are providing an understanding of the evolutionary course of the visual process with new comparative/phylogenetic analyses of molecular and functional mechanisms of photoreception (Terakita, 2005).
Therefore, "simple" animal models, in which the homologues of the major signaling pathways can be better studied, have proved to be useful tools for investigation. By a small selection of relevant examples, this module will survey molecular, functional and behavioral issues typical of invertebrate non-visual photoreception. Among others, we outline some facets of non-visual photoreception of the cnidarian Hydra.
Hydra (Cnidaria, Hydrozoa) is the first metazoan that shows a nervous system, although primitive and structured as a nervous net, and being eyeless it exhibits only non-visual photoreception (Taddei-Ferretti and Musio, 2000). In this animal model, we have identified for the first time a rhodopsin-like protein, and we are unraveling molecular issues regarding the identification and the photic regulation of its opsin photopigments (Musio et al., 2001; Santillo et al., 2006).
Finally, similarities and differences with vertebrate visual and non-visual photoreception (especially between the opsin photopigment families) will be discussed, trying to stress common strategies for light-detection and photosignaling in Metazoa.
2. Chronology and Principles of NVP Terminology
As occurs in all fast growing fields, NVP undergoes a continuous changing of nomenclature strictly related to the update of theoretical concepts and practical aspects. Early attempts to systematize a proper classification and terminology of NVP issues date to Millot (1957), later formalized in Millot (1968, 1978). In those cases, a diffuse non-image-forming photoreception was reported as generalized or dermal light sensitivity for several eyeless or blinded invertebrates showing behavioral actions in response to the direction and the intensity of light (photokinesis, phototaxis).
In the 1960's and 1970's, deep photosensitive cells were identified, also in species having eyes, and mainly located in ganglia or tissues belonging to the central nervous system. They were defined as extraocular photoreceptors, or were assigned to the extraretinal photoreceptor system (Wolken and Mogus, 1981; Wolken, 1988). The same definitions were used in lower vertebrates, where light-sensitivity is also performed by neural structures outside eyes or retinal districts, like the pineal gland or the frontal organ of amphibians.
At that time, the best formalization of NVP terms in invertebrates came from Yoshida (1979), who established the definition of extraocular photoreception based on the sub-classification of dermal, neural and neuronal photosensitivity, including unorganized and organized photoreceptive cells in species with or without primitive or obvious eye-like structures. The classification of extraocular photoreception (with its functional synonym "extraretinal") has been accepted as the standard, and is widely accepted by several authors for both invertebrates and vertebrates (Wolken, 1995).
After the discovery in vertebrates of retinal ipRGCs (that can be considered extraocular in terms of their functional properties, and not on the basis of their morphological localization), a general consensus has been reached for NVP terminology. Nowadays, extraocular (or extraretinal) photosensitive cells are termed non-visual photoreceptors or non-image-forming in invertebrates, and as non-image-forming or non-rod non-cone photoreceptors in vertebrates.
3. Photobehavioral Issues of NVP
Invertebrates exhibit distinct and precise behavioral responses that are mainly associated with the NVP system. Behavioral reactions can be considered to belong to the reflex or to the kinetic type. In some cases, responses elicited by NVP interact with other behavioral activities, of which the entrainment of circadian rhythm is the best studied.
3a. Reflex Responses. The shadow reaction is the most prevailing among many types of reflex responses. The shadow reaction is elicited by a light-on light-off stimulus, directed over a portion of the animal body, and it is detected as a withdrawal or an expansion of the animal body upon illumination. Several reflex responses trigger a phototactic reaction (see below), and require the integration and coordination of a nervous element for their effectiveness.
A useful approach to the study the shadow reflex is to search for the electrical correlates originating from dermal receptors after the removal of the eyes. Accordingly, in the bivalve mollusk, Lima scabra (Mpitsos, 1973), small spikes occur during an off-response, representing activities of the small nerve fibers originated from dermal photoreceptors. Many invertebrates show behavioral responses to visible light even without localized photoreceptors. This occurs mainly in Cnidaria, which show dermatoptic responses, which are also governed by multimodal mechanisms.
The sea anemone, Metridium senile, is a particular case in which the muscle tissue is directly photosensitive upon illumination by blue light (North, 1957). The study of the photic response in the sea anemone, Calamactis praelongus (Marks, 1976), succeeded in distinguishing two types of response mechanisms: one local, due to non-nervous contractions of circular and parietal muscles, the second one is extensive due to neurally coordinated responses. Thus, light is either perceived by sensory cells that are connected to the muscles responsible for contractions in the former case, or by the muscles themselves in the latter one. In this regard, direct muscle photosensitivity has been recently reported in the heart of the isopod crustacean, Ligia exotica (Miyamoto et al., 2006).
Sawyer et al. (1994) have examined and quantified the photoresponse of Anthopleura elegantissima, thereby dissecting the neurophysiological activities of the cellular subsystems underlying the response. They demonstrated that photoreception takes place within sensory cells in a local nerve net, and the photic information is transmitted to the muscles, which are also light-sensitive. The neural correlates of photobehavior in A. elegantissima resemble those of C. praelongus, reported above. In both animals, the effect of irradiance on the activity frequency in the conducting systems may depend on the behavioral state of the anemone. Intrinsic activity is used to maintain body shape, and external stimuli are coded by changes in activity in the relevant conducting system.
3b. Kinetic Reactions. In most instances,, dermal sensitivity appears to be associated with phototactic responses. Such behavioral responses enable the animal to withdraw to a region of higher or lower light intensity and appear to be related to animal survival.
Typical direct tactic photoresponses are shown by Protozoa and other microorganisms (Lenci et al., 1991; Sgarbossa et al., 2002). However, examples regarding this phylum will be not covered in this module, as these organisms represent a case in which a single cell composes a whole organism, and it acts both as effector and receptor. For these reasons Protozoa are not strictly an example of NVP, and we shall consider only cases occurring in multicellular organisms.
The sea squirt, Ciona intestinalis, is of great evolutionary interest because it is a transition site between invertebrates and vertebrates. The adult Ciona is phototropic, and orients itself to the direction of light, accompanied by the opening of the siphons. The animal, having a dermal light sense, also contracts and elongates upon light excitation, even when the siphons are removed. It also has a neural NVP associated with the opening and closing of its siphon (Dilly and Wolken, 1973).
Echinodermata offer a good example of diffuse light-sensitivity due to their particular anatomy. In the brittlestar, Ophioderma, due to the particular structure of its endoskeleton, polarized light produces an oriented behavior acting as a specific signal, and not as a mere directional cue. In addition, animals can discriminate between polarized and unpolarized light (Johnsen, 1994). A primitive form of spatial vision is provided by the echinoid, Echinodema, which is able to locate and move to targets of different sizes, and with different orienting angular widths (Blevins and Johnsen, 2004).
An elegant study on the squid, Lolliguncula, described a "dorsal light reflex", which is part of a light-oriented multimodal sensory system underlying the stabilization of swimming and posture (Preuss and Budelmann, 1995).
Phototactic responses have also been found in insects (Truman, 1976), e.g., the alfalfa weevil, Hypera postica, responds to illumination in the red region of the electromagnetic spectrum by orienting toward the light source. This reaction helps the insect locate its host plant. So, phototaxis represents a good example of interactions between NVP and circadian rhythmic behaviors (see Section 3c).
3c. Interactions between NVP and Other Behavioral Activities. Invertebrate NVP systems appear to be strictly linked to the physiology of biological rhythms and to the entrainment of circadian rhythms; while such systems may work in conjunction with a visual system, they can also function without it (Page, 2009a, b).
A large literature is currently available on the relationships between NVP and the circadian regulation of behavioral activities in several invertebrate models, of which Drosophila (and other arthropoda species) represents a model. In these models, extraretinal photosensitive cells have been identified, and the interaction with the ocular visual system dealing with circadian output has been detailed by multiple approaches (from the molecular to the behavioral-integrative ones). Such studies are worth a longer coverage, and for these reasons the reader could benefit from very recent and comprehensive reviews (Helfrich-Förster et al., 2001; Ashmore and Segal, 2003; Fleissner and Fleissner, 2003; Collins and Blau, 2007; Shiga and Numata, 2007; Vallone et al., 2007).
The daily rhythm of behavioral activity in Aplysia does not require the presence of eyes, though they play a modulatory role on it (Lickey et al., 1976). Block & Smith (1973) identified cerebral photoreceptors that underlie entrainment of circadian rhythms of both locomotion and nervous activity. In isolated ganglia preparations of Aplysia, they detected light responses in most posterior pedal nerves, and pleuro-abdominal connectives determined by photoreceptors located in the cerebral ganglion. Extraocular photoreceptors can also entrain ocular oscillators, and the circadian rhythm of Aplysia overt behavioral activity is in part controlled by both extraocular oscillators and photoreceptors. Furthermore, in some instances the NVP system mediates the response entirely, such as the entrainment of the circadian oscillator in the 6th abdominal ganglion (Lickey et al., 1976).
In the related mollusk, Bulla, basal retinal neurons (BRNs), similar to Aplysia cerebral photoneurons, generate the compound action potential (CAP) output, which is typical of circadian clock activity. BRNs, once dissociated and isolated in cell culture for patch-clamp recordings, express a circadian rhythm of membrane conductance changes (Michel et al., 1993). Contrary to Aplysia photoneurons, Bulla BRNs did not show spontaneous activity in darkness, so their role in producing a circadian rhythm is still questionable.
In the horseshoe crab, Limulus, the tail (telson) has been proposed as a circadian photoreceptor organ. In fact, nerve impulses evoked by light have been recorded from afferent fibers from the tail, even though neither photoreceptors nor their terminations in the central nervous system have yet been identified. This suggests that circadian extraocular photoreceptors, presumably distributed in the animal tail, may cooperate in the timing of visual circadian rhythms, together with the lateral eye, the median ocelli, and the ventral photoreceptors (Renninger and Chamberlain, 1993).
In many marine animals, migration is also a behavior associated to NVP. Migrating rhythms have been studied in the squid, Todarodes sagittatus, and in the turbellarian, Convoluta roscoffensis (Wolken and Mogus, 1981). Convoluta lives in the sand at night during high tide, and emerges onto the surface at low tide during the day time. If the animals are kept in the lab under constant light, vertical migration continues for up to 7 days in synchrony with the tides. The rhythm persists only in constant light, and not in constant darkness.
The NVP system is also related to reproductive development. In the garden slug, Limax maximus, extraocular receptors measure the duration of increasing daylight. This perception of increasing day length results in the secretion of a "maturation hormone" by the brain, and in turn initiates reproductive development (Wolken and Mogus, 1981). In the butterfly, Papilio, light stimulation induces various movements of genitalia, such as penis withdrawal. This stimulation activates two pairs of genitalia NVP sites, which are probably involved in the regulation of copulation behavior (see Section 4c).
4. Functional Mechanisms
This section focuses on the neurophysiological analysis of functional mechanisms responsible for NVP responses. For easy comprehension, examples are reported according to the classification proposed by Yoshida (1979); distinguishing those mediated by elements involved in dermal NVP from those ascribed to elements identified directly as light-sensitive in neural and neuronal NVP systems. Major results obtained in relevant invertebrate species are given in Figure 1.
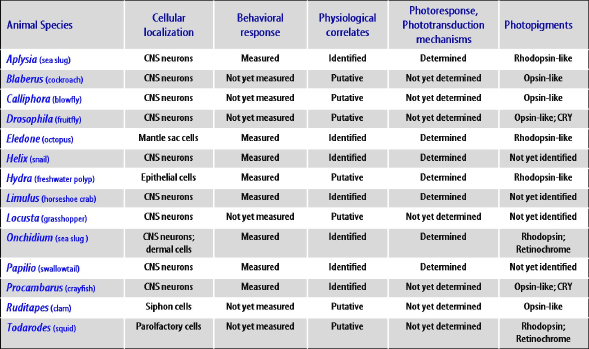
Figure 1. Summary of relevant achievements in popular invertebrate species regarding molecular, cellular and behavioral aspects of non-visual photoreception. See the text for details.
4a. Dermal NVP. Dermal NVP could be considered an evolutive starting point for the neural/neuronal NVP via the diffuse photosensitivity. This is the main reason for the use of primitive organisms to study this sensory capability. Electrophysiological investigation on this kind of photosensitivity are very few, due to experimental difficulties in recording from the whole animal. In other cases (e.g., Hydra), the photosensitivity once ascribed to dermal NVP has been reconsidered to be neuronal NVP.
In the echinoids' nervous system, radial nerves run under each of the five ambulacra, which are branches with rows of podia (tube feet) assuring the animal's fivefold symmetry (Brusca and Brusca, 2003). Radial nerves contain the cell bodies of almost all motor neurons and interneurons, and the axons of sensory neurons located primarily within the ectoderm of podia. Accordingly, the gradations in sensitivity appear to correspond with the distribution of the superficial nervous system. In this way, the dermal light sensitivity is the result of the superficial nerve fibers, which are photosensitive (Yoshida, 1979).
The photobehavior of the cnidarian, Hydra, was early reported as a classical case of dermal light sensitivity, but our recent findings have determined that the Hydra photosensitive mechanisms belong to neuronal NVP, and for these reasons it will be treated elsewhere in this paper (see Sections 4c, and 5aii).
4b. Neural NVP. This kind of photosensitivity may take place within various non-neuronal parts of the nervous system. It could also be assigned to dermal responses, which have been recognized through the functional activity of nervous elements. The location of the photosensitive loci involved is often hard to identify, and the assumption of a nervous source for photoreception should arise from nerve activity recordings, or from the lack of whole neural structures. In this regard, a major problem is encountered when we try to analyze a neural component of NVP. In fact, there is always, with rare exceptions, a degree of uncertainty if the activity arising from the structure under study is directly photo-induced rather than triggered by the activity of associated structures that are the primary site of phototransduction. Furthermore, the putative photosensitivity recorded from nerves can be referred to axonal projections coming from unidentified neuronal elements (central or peripheral). In this case, it should be more appropriate to define it as a neuronal NVP instance (see the example of the butterfly, Papilio, in Section 4c).
As previously reported, the off-response in Aplysia can be recorded from all major branches of the posterior pedal nerves, and further studies of nerve transections have inferred that the photoreceptors may lie in the cerebral ganglion (Block and Smith, 1973; Lickey et al., 1976). The caudal photosensitivity of crayfish is a classical example of neural NVP, although the photosensitivity is not restricted to the caudal region (Wilkens, 1988). In the mollusk, Spisula solidissima, the neural photosensitivity has been recorded from the pallial nerve near the visceral ganglion (Kennedy, 1960). Spontaneous discharges of the proximal end of the nerve were inhibited by illumination on the nerve slightly distal to the visceral ganglion.
The best cases in which the structural correlates for neural photosensitivity have been postulated are seen in mollusks. In Mercenaria mercenaria (Wiederhold et al., 1973), single axon activities of the siphonal nerve were recorded, also under cessation of illumination. Because no photoreceptor cells belonging to the recorded axons were found, the site of phototransduction must have been the axonal branches themselves.
In the pond snail, Lymnaea stagnalis, Chono et al. (2002) reported the electrophysiological correlates of previous behavioral experiments, which proved a positive photo-orientation of the animal even after eye removal. Recordings from the pedal nerves upon light stimulation demonstrated that non-ocular photoresponses were evoked in the foot, and that these responses were independent of ocular photoreception. The lack of identification of proper photoneurons does not provide a precise indication about the efferent/afferent transmission from/to the central nervous system (CNS) ganglia. Light-responsive neurons of pedal ganglia has been reported also in Hermissenda, whose neurons respond to synaptic inputs from other photosensitive cells, considered both as ocular and non-ocular photoreceptors (Jerussi and Alkon, 1981).
4c. Neuronal NVP. In his exemplary review, Yoshida (1979) stated that "Since it is possible to study activities of individual neurons, the works can be more precise than those on any other types of extraocular systems". Recent literature enhances the value of this clear-cut assumption. In the CNS of diverse invertebrates, many neurons have been identified as photosensitive, confirming the usefulness of the electrophysiological approach in ascribing typical issues of neuronal NVP to the system under analysis. Far from being comprehensive, this section attempts to summarize the findings on this kind of NVP.
Cnidarians offer good examples of neuronal NVP, in particular the hydromedusa, Polyorchis, with its photoresponsive neurons in the central nervous system. There is physiological evidence that motoneurons of the swimming pulse system are photosensitive, and are also able to receive synaptic input from other conducting systems (Anderson and Mackie, 1977). These neurons are involved in the control of swimming behavior, and they could represent the neural correlate of the shadow response. However, ten years later, Arkett and Spencer (1986a) demonstrated that the photosensitive locus is not represented by swimming motoneurons, but by an identified neuronal system ("O" system), located in the outer nerve ring and presynaptic to the swimming motoneurons, which send cellular projections (dendritic and/or axonal) to each ocellus. The "O" system shows properties common to the distinctive photoreceptive retinal cell of the invertebrates, the rhabdomeric (or microvillar) photoreceptor (see Section 5b). These results demonstrate the graded nature of the shadow reflex, and the integration of photic information towards the conducting neural nets by the electrical coupling of the neuronal photoreceptors.
Several species of Hydra respond to light by contraction and by tactic movements, the correlates of which can be easily recorded electrophysiologically. The complex mechanisms involved in Hydra light-affected behavior have been elucidated in a classic series of papers (Passano and McCullough, 1962, 1963, 1964, 1965). These authors named the electric correlates of the coordinated contraction as "contraction pulse train", composed of a variable number of "contraction pulses", which are abruptly paused by illumination, and are generated by a pacemaker. Another pacemaker located in the pedal zone elicits "rhythmic potentials", and is inhibited by sudden illumination.
The relationship between photic stimulation and the modulation of the periodic behavior in Hydra have been well studied (reviewed in Taddei-Ferretti and Musio, 1999, 2000; Taddei-Ferretti et al., 2004). The main results include: 1) Hydra's response to a light pulse is a phase shift of the state of bioelectric activity correlated with the periodic shortening-elongation behavior; 2) the direction and absolute value of a phase shift depend on intensity, direction, application phase (along the periodic activity state), and wavelength of the light pulse; 3) repetitive pulses entrain the behavioral cycle; and 4) the period of the behavioral cycle depends on the intensity and wavelength of steady background illumination. Data on the animal behavioral action spectrum and the identification of the photopigment and related molecular issues are reported in Section 5a. More recently, a nitric oxide (NO) pathway has been identified at the neural level by NADPH-diaphorase histochemical labeling. Speculations about possible interactions between the NO pathway and the NVP system have been suggested, although the research is still ongoing (Cristino et al., 2008).
The giant neurons of the visceral ganglion in Aplysia have been most extensively studied. The first evidence on A. fasciata and A. depilans ganglia was reported by Arvanitaki and Chalazonitis (1961) in a seminal paper. These authors found a double mechanism of spiking during on and off light stimulation, indicating that the former is an excitatory event, and the latter is a primary inhibitory one, associated with a heme protein and a carotenoid pigment, respectively. The findings on European species were confirmed on an American species, A. californica. Furthermore, a complete biophysical analysis was performed on these cells leading to the unexpected result of a resemblance between these neurons and atypical ciliated photoreceptor cells of invertebrates that function like vertebrate ciliated photoreceptors (Andresen and Brown, 1979). However, the functional meaning of such marked photosensitivity in these neurons still remains unknown.
The presence of two types of reaction (depolarizing and hyperpolarizing) to illumination has also been found in a group of photosensitive neurons of the subesophageal ganglia of the snail, Helix pomatia (Kartelija et al., 2003), even though the depolarizing response is prevailing. The ionic mechanisms involved and the mediation of the light effect by cyclic nucleotides will be treated in the Section 5b.
The ganglionic complex of the marine pulmonate, Onchidium verruculatum (a closely related species to Aplysia), represents a classical preparation demonstrating neuronal NVP (Hisano et al., 1972). The photosensitive neurons are clustered in the pleuroparietal and in the abdominal ganglia, which provide the execution and the control of the visceral activity of the animal. They have the same structure of other non-photosensitive cells, and do not show any typical morphology of photoreceptor cells such as cilia or microvilli. They react to light with both depolarizing and hyperpolarizing photoresponse, resulting from closing or opening of light-sensitive channels. These activities resemble those of vertebrate and invertebrate retinal photoreceptors, respectively. In this regard, such structurally non-specialized neuronal photoreceptors could represent an ancestral photoreceptor cell for both ciliary and microvillar types. Despite the great amount of data concerning the biophysical mechanisms underlying the photoresponse and visual transduction (see the Section 5), the functional role of these photosensitive neurons remains unclear. It has been suggested that these cells could regulate the transmission of sensory information, mostly of the tactile type, that triggers the reflex underlying the mantle-levating movement. Biophysical details of the Onchidium simple neuronal photosensitive cells are reported in Section 5b.
In the octopus, Eledone cirrhosa, NVP systems have been identified in the epistellar bodies within the mantle cavity on the stellate ganglion. In this case, sufficient light penetrates through the body wall and activates extraocular photoreceptors whose amplitude of the receptor generator potential increases with increasing light intensity (Cobb and Williamson, 1999).
Light detection by neuronal NVP has been identified in the abdominal ganglia of various crustaceans. Despite the fact that in the majority of invertebrates the function of NVP identified in the brain has yet to be interpreted, in Crustacea the functional role of the abdominal NVP systems has been well established.
In Procambarus clarkii, the caudal photoreceptors, located in the 6th abdominal ganglion, are one of the first so-called identified neurons to be described (Wilkens, 1988). The basis of the photic discharge is a relatively slow membrane depolarization, but the ionic mechanisms are not known. The role of these photosensitive neurons is linked to the photonegative behavior of the animal. The functional role is extended to the triggering of the tail reflex, and to the walking behavior of the animal. Leg movements and abdominal motor pattern were evoked by repetitive stimulation of a single caudal photoreceptor at frequencies normally achieved during the cell's response to light (Simon and Edwards, 1990). These results confirmed the link between caudal photoreceptor response and the abdominal motor pattern, and strengthened the link between the caudal photoreceptor response and the backward walking.
Furthermore, the activity of brain NVP systems has been examined in insects by means of pharmacological analysis. In the locust, Locusta migratoria, paired vasopressin-like immunoreactive neurons (VPLI) of the suboesophageal ganglion receive synaptic excitation from extraocular photoreceptors via cholinergic descendent interneurons (DI) (Baines and Bacon, 1994). The excitatory drive to VPLI neurons occurs only in darkness, and is absent in the light; this photosensitivity persists also after ablation of compound eyes, optic lobes and ocelli. The NVP system is sensitive to light of 497±7 nm (blue-green), and has an absorption spectrum of a rhodopsin-like pigment (Baines and Bacon, 1994). The transmitter of this NVP system is histamine, indicating that insect NVP systems and the visual system share histamine as the primary photoreceptor transmitter.
A typical case of multiple peripheral NVP of the neuronal type is provided by the genital photoreceptors (despite their particular dermal location) of the butterfly, Papilio xuthus (reviewed in Arikawa, 2001). In this animal, two pairs of photoreceptive sites (four per individual) have been identified in the pigmented cuticle of the genitalia. The photoresponse of the genital photoreceptors can be recorded from the branches of two abdominal nerves (N5 and N6) that contain the axons of the extraocular photoreceptors. Each nerve shows a sustained train of single spikes to the ganglion under light flash stimulation. Though appearing as separate eyespots, the four extraocular photoreceptors identified are not involved in image-forming tasks, but they simply function as detector of the light covering the body surface in order to regulate copulating behavior.
Finally, the horseshoe crab, Limulus polyphemus, offers a fascinating multiple array of ocular and extraocular structures, which cooperate in both the visual process and the circadian regulation of overall behavior (Battelle, 2006). Extraocular photoneurons thus far identified include photoreceptors adjacent to the optic ganglia, photoreceptors in the median optic nerves, and photoreceptors in the telson. Recently, Mori et al. (2004) found new photosensitive neurons in the opisthosomal ventral nerve cord that may modulate the activity of visceral organs and gill plates upon light stimulation.
However, the classical Limulus extraocular photosensory neurons are the ventral nerve photoreceptors (VNP) classified by Yoshida (1979) as a type of "internally scattered photoreceptive cells" associated with the CNS and not solely restricted to the epidermal surface. The study of VNP light-induced physiology was pioneered by the investigations of Millecchia and Mauro (1969a, b). More recent findings reporting the biophysics of the visual transduction of VNP, related also to new findings about cell structure and molecular issues of its photopigment, will be disussed in Section 5b.
In spite of a great amount of work elucidating phototransductive mechanisms, the functional role of VNP in Limulus behavior remains unclear. The VNP could be involved in the downward or upward movement of the tail at light-on or -off, respectively (Wasserman, 1973). The combined action of tail NVP, lateral eye and VNP, among others, could supply the timekeeping for visual circadian rhythms (Renninger and Chamberlain, 1993), as previously reported in Section 3c.
5. Biophysical Processes
The investigation of biophysical mechanisms can provide essential information about the functional processes underlying NVP. The behavioral analysis and other experimental approaches do not always grant specific evidence for a decisive relationship between light-detection and effector (either a behavior or a cell activity) by the NVP system under analysis. In recent years, the impressive development of molecular and cellular biology and biochemistry, combined with the refinement of advanced electrophysiological techniques, has provided useful tools to investigate the biophysical nature of the elements involved in the photoreceptive (e.g., photopigments) and phototransductive process (e.g., light-sensitive ion channels, second messengers, Ca++ role) involved in NVP.
5a. Photoreceptor Pigments. The identification and the characterization of the photopigment involved in the NVP system is quite difficult, particularly as regards the dermal and the diffuse photosensitivity. In invertebrates, in most cases photopigments have been neither isolated from the photoreceptor sites nor characterized. The identification of any has come primarily from behavioral action spectra and electrophysiological measurements of spectral sensitivity. In contrast, in many vertebrate species, after the discovery of melanopsin (Provencio et al., 1998), several so-called "novel opsins" have been identified, localized and characterized leading, to a new and exciting molecular scenario, which also extends to invertebrate species.
i. Pigment Spectral Sensitivity. Early comparisons between the behavioral action spectra and single NVP cell spectral sensitivity with spectra of known photoreceptor molecules has indicated similarities with the visual pigment rhodopsin. The recent discovery of non-visual novel opsins in vertebrates and invertebrates led us to assume that those similarities are consistent with a basic opsin-based pigment, instead of a rhodopsin.
Previous studies based their assumptions on spectral sensitivity, considering it as the variation of known behavior parameters or cell activities upon stimulation with different wavelengths of light. In most cases, the maximum sensitivity was found between 460 and 500 nm (responses also occurred in the 550-900 nm range). The data currently available indicate that usually the photoreceptor pigment is a carotenoid. In deep-sea cephalopods, rhodopsin has also been extracted (see below). In several cases, a heme or porphyrin pigment molecule has been implicated and identified. However, too few pigments have been extracted from receptor sites belonging to NVP systems.
In Hydra, a red blindness has been found (Passano and McCullough, 1965), and the behavioral action spectrum has been elucidated (reviewed in Taddei-Ferretti and Musio, 2000). Measuring the bioelectric pulses correlated to the body responses, they found two opposite peaks of responses around 450 nm and 550 nm, corresponding to the maximum and minimum duration of the behavioral sequence in undisturbed conditions. Hydra also represents an interesting case in which the periodic behavior can be modulated by different chromatic conditions of stimulation, and natural background illumination (Taddei-Ferretti et al., 1992).
The sea anemone, Anthopleura, shows a flexion toward the rim of the oral disk and a retraction from extended position upon exposure to wavelengths of 250-400 nm and 340-600 nm, respectively (Clark and Kimeldorf, 1970). The action spectrum for the shadow response of cilia of larvae of the sponge, Reniera, was determined by Leys et al. (2002). The authors described a broader peak at 440 nm, due to absorption in either a flavin or a carotenoid, and a smaller peak at 600 nm due to the absorption by a putative opsin-like pigment.
The photosensitive neurons of Onchidium and Aplysia are orange pigmented, and the spectral sensitivity indicates the presence of a hemoprotein and a carotenoid. In cephalopods, a photopigment was spectroscopically and biochemically identified, to be the same as rhodopsin in the eye, in epistellar body and parolfactory vesicles, respectively, in Eledone and in Loligo. In the squid, Todarodes, the first quantitative study on mollusks found that retinochrome exists together with rhodopsin, in parolfactory vesicles, with peaks at 480 and 490 nm, respectively (Hara and Hara, 1980).
ii. Identification of Photopigments (with some hints on their molecular evolution). The searching for photopigments triggering non-image-forming photoreception is a new challenging field in vision research. Several reports regard the localization and function of such pigments in NVP systems in vertebrates (Foster and Hankins, 2002), while still few accounts are available on invertebrates (Santillo et al., 2006).
Anyway, the NVP photopigments identified so far are all referred to as opsin-like proteins (Terakita, 2005). Recently, a putative role for the blue-light flavoprotein cryptochrome (CRY) as the NVP photopigment in the light-regulated behavior of insects (Emery et al., 2000), crustaceans (Fanjul-Moles et al., 2004), and corals (Levy et al., 2007) is emerging. Nevertheless, a conserved photoreceptive role for CRY in vertebrate eyes has been discovered recently in chick iris, whose constriction to light ex vivo depends on CRY rather than on opsin activity (Tu et al., 2004).
Despite inter- and intra-species functional differences, molecular genetics approaches have identified about 1,000 opsins belonging to both vertebrates and invertebrates. The updated molecular phylogenetic tree of animal opsins (identified so far) shows seven subfamilies corresponding to a functional classification of opsins based on specific G-protein type that links each proper opsin receptor (Terakita, 2005).
The opsin subfamilies (Figure 2) include:
1) vertebrate visual (T-coupled) and non-visual opsins;The functional diversity of opsins, and their localization, indicates that higher percentages (about 70%) of these proteins deal with non-image forming systems (Figure 2).
2) encephalopsin/tmt-opsins;
3) Gq-coupled opsins/melanopsins;
4) Go-coupled opsins;
5) neuropsins;
6) peropsins;
7) RGR retinal photoisomerases.
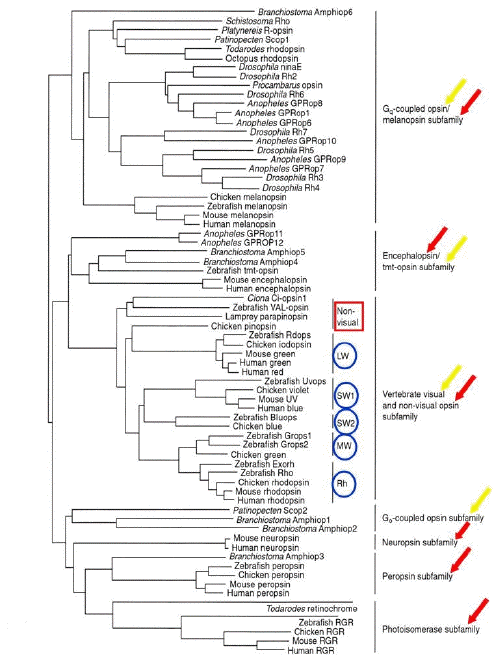
Figure 2. Molecular phylogenetic tree of the animal opsin family (by the neighbor-joining method). Canonical visual opsins and novel non-visual opsins are indicated, respectively, with yellow and red arrows. Blue circled acronyms indicate retinal photopigments of vertebrates; LW, MW, SW1, SW2, respectively, long-, medium-, type 1 short- , type 2 short-wavelength opsin of cones, and Rh, rhodopsin of rods. (Modified from Terakita, 2005).
Recent findings on the molecular evolution of novel non-visual opsins suggest a strict relationship of the NVP process with canonical vision, which could have occurred in lower organisms of the animal phylogenetic tree. In this regard, the identification of novel opsins suggests that the history of visual pigments is strictly connected with the evolution of photoreceptors and eyes (Nilsson, 2005). In particular, there is strong evidence that opsins evolved according to the main evolutionary lineages of animal visual cells, the ciliary and the rhabdomeric (or microvillar) photoreceptors (see Section 5b). However, although those aspects are not strictly related to the main topics of this paper, some accounts related to NVP will be given.
In the primitive eyeless metazoan, Hydra, we first identified an opsin-like protein (by polyclonal antibodies against squid rhodopsin) probably localized in epidermal sensory nervous cells (Musio et al., 2001)
(Figure 3).
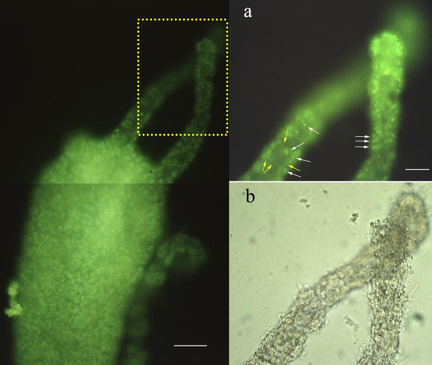
Figure 3. Immunofluorescence localization of a rhodopsin-like protein in Hydra vulgaris.
Left: The distribution pattern of the fluorescent cells is restricted to the ectodermal surface of the animal. Dotted inset is magnified on top right figure. Bar: 0.05 mm.
Right: (a) Higher magnification of tentacules allows the visualization of the epidermal sensory cell bodies (white arrows), and the axonal processes constituting the nerve-net (yellow arrows): (b) Bright-field micrograph of (a). Bar: 0.1 mm. (Modified from Musio et al., 2001).
Molecular approaches, using partial sequences of opsin genes available in the GeneBank, provided us with preliminary evidence of a possible coexistence of putative visual and non-visual opsins in a primitive animal (Santillo et al., 2006). Hopefully, interesting results will be forthcoming on the regulation of opsin gene expression exerted by diurnal and circadian rhythms in Hydra (Santillo et al., 2009).
More recently, Suga et al. (2008) reported in jellyfish as many as eighteen opsins in Cladonema, and two in Podocoryne. Their expression patterns suggest two possible functions: a role in vision by the eye, and the other involved in the timing control of oogenesis or spawning process, possibly in cooperation with cryptochromes. Furthermore, recent papers accounting for the molecular evolution of opsin visual pigments have reported the identification of multiple classes of opsins in Cnidaria (Plachetzki et al., 2007; Alvarez, 2008).
In the brain of four species of lepidopterans, three kinds of spectrally distinct opsins have been reported outside of the retina; UV and blue opsins, which are restricted to adult stemmata (where melatonin is expressed together with opsins), and long-wavelength (LW) opsins, which are specific for dorsal and ventral photosensitive neurons of the optic lobes. These opsins provide the extraretinal detection of ambient light variations, and are involved in the neuroendocrine output mediated by melatonin, underlying the entrainment of circadian and/or photoperiodic rhythms (Lampel et al., 2005).
Arendt and coworkers (2004) have found that in the ragworm, Platinereis, the coexistence of rhabdomeric photoreceptors in the eyes (for phototaxis), and ciliary photosensitive cells in the brain (for entrainment of biological clocks). The latter (referred to as NVP cells) use an opsin closely related to vertebrate rod and cone opsins. A recent study in the honey bee, Apis mellifera, has revealed that a ciliary opsin, called pteropsin, is expressed in the brain of this species, indicating the presence of a vertebrate-like light-detecting system in insects (Velarde et al., 2005).
The hypothesis that rhabdomeric invertebrate photoreceptors and photosensitive ganglion cells could have common molecular machinery has been put forward by Koyanagi et al. (2005). They used the cephalocordate, Ampioxus, the invertebrate closest to a vertebrate that has rhabdomeric photoreceptors for non-visual function. These authors found that the amphioxus homolog of melanopsin was contained in rhabdomeric photoreceptors. It shows the biochemical and photochemical properties of the visual rhodopsins, similar to those of classic rhabdomeric photoreceptors common to higher invertebrates. Ultimate electrophysiological findings in melanopsin-expressing photoreceptors of Amphioxus support the above hypothesis about a link between ancestral rhabdomeric photosensitive cells of prebilaterians, and the circadian photoreceptors of higher vertebrates (Gomez del Pilar et al., 2009).
More recently, a striking molecular feature of melanopsin has been reported to be phylogenetically close to the visual pigments of invertebrates. In particular, Terakita et al. (2008) successfully expressed and purified UV and blue light-sensitive visual pigments of honeybee, as well as of amphioxus melanopsin. They reported similar molecular properties between melanopsin and Gq-coupled visual pigments, although these photopigments serve different visual functions.
5b. Light-Sensitive Channels and Phototransduction Cascade. Invertebrates show a great variety of eyes and retinal structural patterns constituted by microvillar photoreceptors, with very few ciliary exceptions (Eakin RM, 1972). Rhabdomeric photoreceptors are carachterized by wide finger-like invaginations of the cellular membrane, called microvilli, which are variously arranged and contain visual pigments inside (Figure 4).
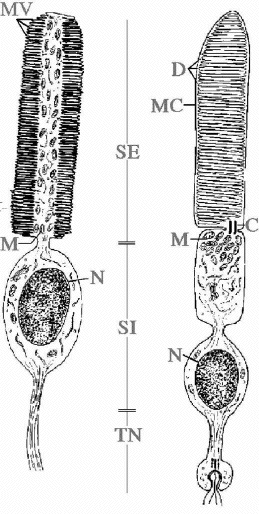
Figure 4. Scheme of a rhabdomeric (left) and a ciliary (right) photoreeptor. C, cilium; D, disks; M, mitocondria; MC, cellular membrane; MV, microvilli; N, nucleus; SE, outer segment; SI, inner segment; TN, nervous termination (original drawing, courtesy of Prof. Celina Bedini, University of Pisa).
In spite of the functional development of optical solutions, vertebrates share a substantially conserved structural scheme. The image-forming photosensitive elements are constituted by retinal ciliary photoreceptors, rods and cones (Cohen, 1972). Ciliary photoreceptors show a more regular structure, being entirely of ciliary type. They are carachterized by flattened disks or sacks containing photopigments, which originated from the invagination of the cellular membrane (Figure 4).
The two main evolutionary lineages of visual cells, ciliary and microvillar (rhabdomeric), have different functional properties of visual excitation, although in both the transduction mechanism is characterized by a G-protein-coupled cascade mediated by a second messenger acting on the gating of light-dependent ion channels (Figure 5).
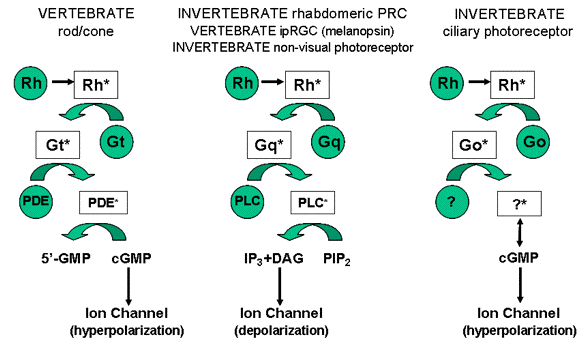
Figure 5. Schematic drawing of the different phototransduction cascades occurring in classical and non-visual photoreceptors in both vertebrates and invertebrates. Green circles represent the elements of the pathway "G-protein-coupled receptor→
G-protein→effector enzyme" shared by both photoreceptor types. Abbreviations: Rh, photopigment; Rh*, activated photopigment; Gt, Gq, Go, respectively G protein subunit t, q, o and their activated forms Gt*, Gq*, Go*; PDE, phosphodiesterase; PDE*, activated phosphodiesterase; PLC, phospholipase C; PLC*, activated phospholipase C; 5'-GMP, 5'- guanosine monophosphate; cGMP, cyclic guanosine monophosphate; IP3, inositol 1,4,5-triphosphate; DAG, diacylglycerol; PIP2, phosphatidyl inositol 4,5 bisphosphate. (Modified from Santillo et al., 2006).
Due to ancillary and, above all, to new advanced electrophysiological techniques, the study of functional properties of photoresponse in invertebrate photoreceptors is orienting towards the "single cell approach" (Musio, 1996, 2001). This kind of approach is fruitful when a given cell has already been identified as a photoreceptor, or is used to discover new examples of photosensitivity (Nasi et al., 2000). The best studied NVP models by means of this approach are those cells identified as neuronal photoreceptors, or those cells belonging to the central nervous system with the soma located outside the brain in peripheral sensory regions (Musio, 1996, 2001).
Important electrophysiological data on the mechanisms of the intracellular signaling cascade in the melanopsin-containing retinal ganglion cells (ipGRCs) of vertebrates have been reported. These papers show that melanopsin phototransduction resembles the responses of invertebrate photoreceptors, and not the responses seen in vertebrate classical photoreceptors (rods and cones) (Isoldi et al., 2005; Contin et al., 2006; Graham et al., 2008) (see Figure 5). Unfortunately, electrophysiological investigations on the phototransduction chain in NVP cells of invertebrates are still limited. However, relevant examples can be gathered from mollusks and Limulus.
Since the middle of the 1980's, Gotow and coworkers have extensively studied the ionic mechanisms involved in the photoresponse of the neuronal photoreceptors of the mollusk, Onchidium (reviewed in Gotow and Nishi, 2008). Identified abdominal neurons (A-P-1) respond to light with a decrease of K+ conductance, due to the closing of light-sensitive channels associated with membrane depolarization. This finding resembles, except for the response polarity, the light-induced hyperpolarizing potential in vertebrate photoreceptors that is also induced by a conductance decrease. Instead, differences are evident if the A-P-1 ionic behavior is compared to the same photoresponse in ocular and extraocular photoreceptors of other invertebrates. In fact, it is known that most invertebrate ocular photoreceptors respond to light with a membrane depolarization produced by an increase of membrane conductance to Na+ and K+ ions following the opening of light-sensitive channels. Similarly, an increase of conductance occurs also in extraocular photosensitive neurons in the abdominal ganglion of Aplysia (Andresen and Brown, 1979), even though in these neurons the receptor potential is hyperpolarizing, analogous to that in invertebrate ciliary ocular photoreceptors (Gomez and Nasi, 1994).
The photoresponse of Onchidium A-P-1 resembles that of vertebrate photoreceptors, as regards the role of internal messenger. Injection of cyclic guanosine monophosphate (cGMP) in the dark produces an outward current, associated with an increase of conductance, which is suppressed by illumination (suggesting a hydrolysis of cGMP by light). Thus, cGMP mediates Onchidium A-P-1 phototransduction, because the photoresponse is the suppression of the cGMP-induced current. Thus, light activates a phosphodiesterase that reduces cGMP, as in vertebrate photoreceptors (Koutalos et al., 2001). Furthermore, the photocurrent is amplified by the pressure-injection of inositol 1,4,5-triphosphate (IP3), indicating also a role of this messenger in the visual cascade. In this way, the light-sensitive channels of the extraocular photoreceptors seem to be regulated by cGMP in the dark, and by IP3 in the light. These findings have been confirmed by Gotow et al. (1994), who first recorded a light-sensitive ion channel belonging to a neuronal photoreceptor. By means of cell-attached patch clamp configuration, they found a light-sensitive K+ channel that is active (opened) during dark, and is closed upon light. In the inside-out configuration, a channel that appeared to be the same as the light-sensitive channel was activated (opened) by the application of cGMP.
The photosensitive neurons in the left parietal ganglion of the snail, Helix, provide another well-studied model of ionic mechanisms underlying light detection (Kartelija et al., 2003). In these cells, the light produces a slow inward current associated with a decrement of slope conductance. This light-induced current is due to the suppression of K2 conductance, and the addition of an internal concentration of cGMP mimics the effect of light. In fact, the trend of light-sensitive and cGMP-induced currents follows a similar course, and shows a common reversal potential. This differs from the Onchidium photosensitive neurons, because in the former case, cGMP acts to produce an outward current that is suppressed by light.
In the octopus, Eledone, it has been demonstrated that extraocular photoreceptors, termed "epistellar bodies", located inside the mantle sac, depolarize upon an increase in illumination due to an increase in cell membrane conductance (Cobb and Williamson, 1999). The current flowing through the depolarizing conductance is mainly carried by Na+. This study indicates that octopus extraocular photoreceptor cells are comparable in their light-induced depolarization (and the underlying ionic phototransduction mechanism) with those already reported for other invertebrate rhabdomeric photoreceptor cells (Nasi et al., 2000).
Apart from its evolutionary value in the development of the photoreceptive function, the Limulus ventral nerve photoreceptor (VNP) is certainly the well-established invertebrate model (among those currently used) to investigate light-induced biophysical processes. Since the pioneering works of Millecchia and Mauro (1969a, b), a huge number of studies carried out by several authors have revealed the exceptional suitability of the Limulus VNP to quantitative electrophysiology and biochemical approaches. For the convenience of the reader, a very brief summary of the main biophysical characteristics of the Limulus VNP is given below, with the recommendation that the reader check these excellent reviews for detailed results and references (Nagy, 1991; Stieve and Nagy, 1997).
The function of Limulus VNP appears very effective, due to the synergic action of light- and voltage-activated conductances. Voltage-clamp recordings showed three different light-activated conductances (which act together to shape three different components to the receptor macroscopic current), and four voltage-activated conductances. These components recover with different rates, and have different reversal potentials and behavioral kinetics as well, indicating different excitation mechanisms.
In this respect, current data propose three transduction pathways leading to different terminal transmitters: 1) An unknown transmitter produced by the Phospholipase C (PLC*)→ IP3 → Ca2+ enzymatic cascade; 2) cGMP; and 3) cyclic adenosine monophosphate (cAMP). The starting points of these chains, after the triggering by photon absorption, could be different G proteins activated by the same metarhodopsin molecule, even though the presence of other types of metarhodopsin cannot be excluded.
Calcium ions also play a crucial role in the Limulus VNP phototransductive process. Initially, calcium was supposed as the terminal transmitter produced by the IP3 enzymatic cascade, while a strong injection of Ca2+ in VNP causes a conductance increase. Further evidence set aside this idea, and it favors Ca2+ as the messenger for the adaptation process. Although free Ca2+ ions do not pass through the light-sensitive channels, they are important in the activation of these channels, establishing and regulating feedbacks of the enzymatic cascades underlying the production of transmitters, and the intracellular calcium level. Recent additions to the VNP phototransduction cascade have been provided by Garger et al. (2004), who conclude that guanylate cyclase is downstream of the IP3-induced Ca2+ release, and is the final enzymatic step of the excitation cascade producing a rise in cGMP, which opens cyclic nucleotide-gated ion channels (CNCG) in the plasma membrane.
In conclusion, there are still few available examples to depict a common phototransductive cascade by invertebrate NVP cells. A large amount of data is needed to: a) compare invertebrate and vertebrate NVP cell physiology; and b) verify the ultimate findings on vertebrate NVP cell whose physiology seems to be very close to that of invertebrate visual photoreceptors.
In fact, previous studies have shown that melanopsin belongs to the orthology group of rhabdomeric opsins, coupling possibly to an invertebrate-like phototransduction cascade. This is indicated by an IP3-based visual cascade triggered by melanopsin in cultured Xenopus melanophore systems (Isoldi et al., 2005), and by the co-localization of melanopsin with Gq, but not with other G-proteins, such as Gs, Gt, and Go (Koyanagi and Terakita, 2008).
The involvement of an invertebrate-like (rhabdomeric) phototransduction cascade in melanopsin-containing photoreceptors has been recently identified in non-visual retinal ganglion cells of chicken (Contin et al., 2006), and in intrinsically photosensitive retinal ganglion cells (ipRGCs), and in cultured intrinsically photosensitive retinal ganglion cells (ipRGCs) of the rat (Graham et al., 2008). Finally, identified elements of the phototransduction cascade of visual and non-visual photoreceptors of invertebrates species cited in the present paper are reported for comparison in Figure 6.

Figure 6. Detail of the key-players involved in the phototransduction of visual and non-visual photoreceptors in some invertebrates. On the bottom, comparison with image-forming and extraretinal photoreceptors of vertebrates is given. Each single row should be read from left to right according to the temporal order of the functional events. The up arrows and down arrows, respectively, mean increase and decrease of the intracellular concentration of the chemical substance. For details and abbreviations see Section 5b.
6. Concluding Remarks and Future Directions
There is no doubt that the findings obtained in the last decade on molecular, cellular and functional properties of non-visual photoreception are bringing this research field to a new height. However, several issues remain to be unraveled, and more animal models are needed to understand them. Above all, the study of the molecular evolution of the novel opsins shows a need for discovering the evolution of photoreceptors, and definitely of visual function. Also, the comparative physiology of the non-visual photoreceptor may shed light on the course of the phototransduction cascade along the phylogenetic tree. Interesting scenarios have been recently proposed (Arendt et al., 2004; Koyanagi and Terakita, 2008), but need further confirmation.
From the evolutionary point of view, it has been suggested that NVP, through undifferentiated single photosensitive cells, constitutes the first step towards the complex organization of photoreceptive elements clustered into cup-like ocelli, and later into eyes. On the other hand, it should be considered: 1) the persistence of NVP also in recent phyla with developed ocular photoreception, 2) its widespread occurrence in the animal kingdom, in some cases with coexistence of different types of NVP in the same animal, and 3) the important role played by NVP (with or without a direct link with retinal photoreception) in several processes of temporal physiology. All together these facts exclude the possibility of considering NVP as a primitive evolutionary step in lower Metazoa, and an evolutionary relic in higher phyla. On the contrary, they suggest a polyphyletic route (i.e., evolved independently many times, and not from a common single ancestor) of the photoreceptor function, which has evolved and split with a high survival value to serve different visual tasks, such as image-forming and circadian vision.
The identification of novel opsins in a wide number of species, above all for invertebrates, their molecular evolution and phylogenetic analysis will help to provide clear answers. Of high priority should be the effort to untangle the evolutionary relationship between invertebrate non-visual cells and photosensitive ganglion cells, since it concerns the putative common molecular and photochemical strategies of phototransduction.
From a functional point of view, recent studies in vertebrates including mammals, stress the crucial role of NVP, which seems to parallel and integrate the image-forming process. Intrinsically light sensitive melanopsin-expressing ganglion cells provide retinal input, via the retino-hypothalamic tract, to the hypothalamic suprachiasmatic nucleus (SCN) that synchronizes the SCN circadian oscillator to the external day/night cycle (Warren et al., 2003). In addition, conventional ganglion cells also innervate the SCN, indicating that the rod/cone system of photoreceptors may provide signals to the SCN circadian system independent of intrinsically light-sensitive melanopsin ganglion cells (Sollars et al., 2003). Surprisingly, in primate melanopsin-expressing retinal ganglion cells, which project axon pathways to the lateral geniculate nuclei (the brain structure acting as a relay station for image-forming information), send irradiance and color information previously gathered by the rods and cones. Thus, image-forming and non-image-forming systems are merged, and melanopsin may contribute to conscious visual perception (Dacey et al., 2005).
Thanks to the peculiar characteristics at the cellular levels, functional studies on invertebrates should be mainly directed to deepen the modulatory role of NVP on temporal physiology and photoentrainment of circadian rhythms. In addition, due to the close link between non-visual extraocular photoreception and circadian regulation, the study of NVP could be extended to pharmacological and clinical aspects. In this regard, the recent demonstration of a direct role of melanopsin in mediating the photic regulation of sleep (Lupi et al., 2008), the melanopsin signaling systems could be a potentially a new pharmacological target for the selective manipulation of sleep and arousal states. Melanopsin as a sleep modulator (Tsai et al., 2009), and as a player in altered human sleep phenotypes has been reported (Roecklein et al., 2008).
In summary, we can assert that non-visual photoreception is a renewed, fast-growing and deep-rooted area of photosensory biology, and visual neuroscience. The study of NVP can provide crucial details on the evolution of visual systems. Moreover, it can be a poweful tool to investigate important clinical implications for several pathological conditions (e.g., blindness, deficits in the circadian physiology, and sleep/wake timing), whose defects nowadays cannot be excluded from defects occurring in the non-visual phototransduction pathway(s).
REFERENCES
Alvarez CE. 2008. On the origins of arrestin and rhodopsin. BMC Evolutionary Biology 8:222
Anderson PAV, Mackie GO. 1977. Electrically coupled, photosensitive neurons control swimming in a jellyfish. Science 197:186-188.
Andresen MC, Brown AM. 1979. Photoresponses of a extraretinal photoreceptor in Aplysia. J. Physiol 287:26-282.
Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. 2004. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 306:869-871.
Arikawa K. 2001. What do butterflies "see" with their genitalia? Biological function of the genital photoreceptors of the swallowtail butterfly, , In Vision: the approach of biophysics and neurosciences (Musio C, ed.) World Scientific, Singapore pp. 119-130.
Arkett SA, Spencer AN. 1986a. Neuronal mechanism of a hydromedusan shadow reflex. I. Identified reflex components and sequence of events. J. Comp. Physiol. A 159:201-213.
Arkett SA, Spencer AN. 1986b. Neuronal mechanism of a hydromedusan shadow reflex. II. Graded responses of reflex components, possible mechanism of photic integration, and functional significance. J. Comp. Physiol. A 159:215-225.
Arvanitaki A, Chalazonitis N. 1961. Excitatory and inhibitory processes initiated by light and infra-red radiations in single identifiable nerve cells (giant ganglion cells of Aplysia), In: Nervous Inhibition (Florey E, ed.) Pergamon, Oxford pp. 194-231.
Ashmore LJ, Sehgal A. 2003. A fly's eye view of circadian entrainment. J Biol Rhythms. 18:206-16.
Baines RA, Bacon JP. 1994. Pharmacological analysis of the cholinergic input to the locust VPLI neuron from an extraocular photoreceptor system. J. Neurophysiol. 72:2864-2874.
Battelle BA. 2006. The eyes of Limulus polyphemus (Xiphosura, Chelicerata) and their afferent and efferent projections. Arthropod Structure & Development 35:261-274.
Bertolucci C, Foà A. 2004. Extraocular photoreception and circadian entrainment in nonmammalian vertebrates. Chronobiol Int. 21:501-19.
Blevins E, Johnsen S. 2004. Spatial vision in the echinoid genus Echinometra. J. Exp Biol 207:4249-4253.
Block G, Smith JT. 1973. Cerebral photoreceptors in Aplysia. Comp. Biochem. Physiol. 46A:115-121.
Brusca, R, Brusca, G. 2003. Invertebrates. Sinauer Associates, Sunderland, MA.
Clark ED, Kimeldorf DJ. 1970. Tentacle responses of the sea anemone Anthopleura xanthogrammica to ultraviolet and visible light. Nature 227:856-857.
Chono K, Fujito Y, Ito E. 2002., Non-ocular dermal photoreception in the pond snail Lymnaea stagnalis. Brain Res. 951:107-112.
Cobb CS, Williamson R. 1999. Ionic mechanisms of phototransduction in photoreceptor cells from the epistellar body of the octopus Eledone cirrhosa. J Exp Biol. 202:977-86.
Cohen AI. 1972. Rods and cones. In: Handbook of Sensory Physiology, vol. VII/2 (Fuortes MGF, ed) Springer, Berlin pp. 63-110.
Collins B, Blau J. 2007. Even a stopped clock tells the right time twice a day: circadian timekeeping in Drosophila. Pflugers Arch. 454:857-67.
Contin MA, Verra DM, Guido ME. 2006. An invertebrate-like phototransduction cascade mediates light detection in the chicken retinal ganglion cells. FASEB J. 20(14):2648-50.
Cristino L, Guglielmotti V, Cotugno A, Musio C, Santillo S. 2008. Nitric oxide signaling pathways at neural level in invertebrates: functional implications in cnidarians. Brain Research 1225:17-25.
Dacey DM, Liao H-W, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau K-W, Gamlin PD. 2005. Melanopsinexpressing ganglion cells in primate retina signal color and irradiance and project to the LGN. Nature 433:749-754.
Dilly PN, JJ Wolken. 1973. Studies on the receptors in Ciona intestinalis. IV. The ocellus in the adult. Micron 4:11-29.
Eakin RM. 1972. Structure of invertebrate photoreceptors. In: Handbook of Sensory Physiology, vol.VII/1 (Dartnall HJ, ed) Springer, Berlin pp. 625-684.
Emery P, Stanewsky R, Helfrich-Förster C, Emery-Le M, Hall JC, Rosbash M, 2000. Drosophila CRY is a deep brain circadian photoreceptor. Neuron 26, 493-504.
Fanjul-Moles ML, Escamilla-Chimal EG, Gloria-Soria A, Hernández-Herrera G. 2004. The crayfish Procambarus clarkii CRY shows daily and circadian variation. J Exp Biol 207:1453-1460.
Fleissner G, Fleissner G. 2003. Nonvisual photoreceptors in arthropods with emphasis on their putative role as receptors of natural Zeitgeber stimuli. Chronobiol Int. 20(4):593-616.
Foster RG, Hankins MW. 2002. Non-rod, non-cone photoreception in the vertebrates. Prog. Ret. Eye Res. 21:507-527.
Foster RG, Hankins MW. 2007. Circadian vision. Curr. Biol. 17(17):R746-51.
Foster RG, Helfrich-Förster C. 2001. The regulation of circadian clocks by light in fruitflies and mice. Phil. Trans. R. Soc. B 356:1779-1789.
Fu Y, Liao H-W, Tri H, Do M, Yau K-W. 2005. Non-image-forming ocular photoreception in vertebrates. Curr. Opin. Neurobiol. 15:415-422.
Garger AV, Edwin AR, Lisman JE. 2004. The excitation cascade of Limulus ventral photoreceptors: guanylate cyclase as the link between InsP3-mediated Ca2+ release and the opening of cGMP-gated channels, BMC Neuroscience 5:7.
Gomez M, Nasi E. 1994. The light-sensitive conductance of hyperpolarizing invertebrate photoreceptors: a patch-clamp study. J. Gen Physiol. 103:939-956.
Gomez del Pilar M, Angueyra JM, Nasi E. 2009. Light-transduction in melanopsin-expressing photoreceptors of Amphioxus. PNAS 106:9081-9086
Gotow T, Nishi T. 2008. Simple photoreceptors in some invertebrates: Physiological properties of a new photosensory modality. Brain Research 1225:3-16.
Gotow T, Nishi T, Kijima H. 1994. Single K+ channels closed by light and opened by cyclic GMP in molluscan extra-ocular photoreceptor cells. Brain Res. 662:268-272.
Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. 2008. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol 99:2522-2532.
Guido ME, Carpentieri AR, Garbarino-Pico E. 2002. Circadian phototransduction and the regulation of biological rhythms. Neurochem Res. 27:1473-89.
Hankins MW, Peirson SN, Foster RG. 2008. Melanopsin: an exciting photopigment. Trends Neurosci. 31:27-36.
Hara T, Hara R. 1980. Retinochrome and rhodopsin in the extraocular photoreceptor of the squid, Todarodes. J. Gen. Physiol. 75:1-19.
Helfrich-Förster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. 2001. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron 30:249-261.
Hisano N, Tateda H, Kuwabara M. 1972. Photosensitive neurones in the marine pulmonate mollusc Onchidium verruculatum. J. Exp. Biol. 57:651-660.
Isoldi MC, Rollag MD, Castrucci AM, Provencio I. 2005. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. PNAS 102:1217-21.
Jerussi TP, Alkon DL.1981. Ocular and extraocular responses of identifiable neurons in pedal ganglia of Hermissenda crassicornis. J. Neurophysiol. 46:659-671.
Johnsen S. 1994. Extraocular sensitivity to polarized light in an echinoderm. J. Exp. Biol., 195:281-291.
Kartelija G, Nedeljkovic M, Radenovic L. 2003 Photosensitive neurons in mollusks. Comp Biochem Physiol A Mol Integr Physiol. 134:483-95.
Kennedy D. 1960. Neural photoreception in a lamellibranch mollusc. J. Gen. Physiol. 44:277-299.
Koutalos Y, Nakatani K, Xiong W-H, Yau K-W. 2001. Phototransduction in retinal rods and cones, In: Vision: The approach of biophysics and neurosciences (Musio C, ed.) World Scientific, Singapore pp. 172-183.
Koyanagi M, Terakita A. 2008. Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem Photobiol. 84:1024-30.
Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. 2005. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr. Biol. 15:1065-1069.
Lampel J, Briscoe AD, Wasserthal LT. 2005. Expression of UV-, blue-, long-wavelength-sensitive opsins and melatonin in extraretinal photoreceptors of the optic lobes of hawkmoths. Cell Tissue Res 321:443-458.
Leys SP, Cronin TW, Degnan BM, Marshall JN. 2002, Spectral sensitivity in a sponge larva. J. Comp. Physiol. A 188:199-202
Lenci F, Ghetti F, Colombetti G, Häder D-P, Song P-S, eds. 1991. Biophysics of photoreceptors and photomovements in microorganisms. Plenum, New York, London.
Land MF, Nilsson DE. 2002. Animal Eyes. Oxford University Press, Oxford.
Levy O, Appelbaum L, Leggat W, Gothlif Y, Hayward DC, Miller DJ, Hoegh-Guldberg, O. 2007. Light-responsive cryptochromes from a simple multicellular animal, the coral, Acropora millepora. Science 318: 467-470.
Lickey ME, Block GD, Hudson D, Smith JT. 1976. Circadian oscillators and photoreceptors in the gastropod Aplysia. Photochem. Photobiol. 23:253-273.
Lupi D, Oster H, Thompson S, Foster RG. 2008. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 11:1068-73.
Marks PS. 1976. Nervous control of light responses in the sea anemone Calamactis praelongus. J. Exp. Biol. 65:85-96.
Meyer JR. 1977. Head capsule transmission of long-wavelength light in the Curculionidae. Science 196:524-525.
Michel S, Geusz ME, Zaritsky JJ, Block GD. 1993. Circadian rhythm in membrane conductance expressed in isolated neurons. Science 259:239-41.
Millecchia R, Mauro A. 1969a. The ventral photoreceptor cells of Limulus. II. The basic photoresponse. J. Gen. Physiol. 54:310-330.
Millecchia R, Mauro A. 1969b. The ventral photoreceptor cells of Limulus. III. A voltage clamp study. J. Gen. Physiol. 54:331-351.
Millott N. 1957. Animal photosensitivity, with special reference to eyeless forms. Endeavour 16:19-28.
Millott N. 1968. The dermal light sense. In: Invertebrate photoreceptors (Carthy JD, Newell GE eds) Academic Press, New York, London pp. 1-36.
Millott N. 1978. Extra-ocular photosensitivity. Meadowfield Press, Burham.
Miyamoto H, Horiguchi H, Hariyama T, Takano S, Yamagishi H. 2006. Photosensitive neurogenic heart of the isopod crustacean Ligia exotica. Proc Biol Sci. 273(1600):2535-40.
Mori K, Saito T, Kuramoto T. 2004. Physiological and morphological identification of photosensitive neurons in the opisthosomal ganglia of Limulus polyphemus. Biol. Bull. 207:209-216.
Mpitsos GJ. 1973. Physiology of vision in the mollusk Lima scabra. J. Neurophysiol. 37:371-383.
Musio C. 1996. Application of the patch-clamp technique to photoreceptor cells of the crayfish Orconectes limosus. Ital. J. Zool. 63:135-138.
Musio C. 1997. Extraocular photosensitivity in invertebrates. In: Biophysics of photoreception: Molecular and phototransductive events (Taddei-Ferretti C, ed.) World Scientific, Singapore pp. 245-262.
Musio C. 2001. Patch-clamping solitary visual cells to understand cellular mechanisms of invertebrate phototransduction. In: Vision: The approach of biophysics and neurosciences (Musio C, ed.) World Scientific, Singapore pp. 145-164.
Musio C, Santillo S, Taddei-Ferretti C, Robles LJ, Vismara R, Barsanti L, Gualtieri P. 2001. First identification and localization of a visual pigment in Hydra (Cnidaria, Hydrozoa). J. Comp. Physiol. 187A:79-81.
Nagy K. 1991. Biophysical processes in invertebrate photoreceptors: recent progress and a critical overview based on Limulus photoreceptors. Quart. Rev. Biophys. 24:165-226.
Nasi E, Gomez M, Payne R. 2000. Phototransduction mechanisms in microvillar and ciliary photoreceptors of invertebrates. In: Molecular Mechanisms in Visual Transduction (Hoff AJ, Stavenga D, de Grip WJ, Pugh EN, eds.) Elsevier, Amsterdam pp. 389-448.
Nilsson D-E. 2005. Photoreceptor evolution: ancient siblings serve different tasks. Curr. Biol. 15:R94-96.
North WJ. 1957. Sensitivity to light in the sea anemone Metridium senile (L.). II. Studies on reaction time variability and effects of change in light intensity and temperature. J. Gen. Physiol. 40:715-733.
Page TL. 2009a. Circadian regulation in invertebrates. Encyclopedia of Neuroscience (Elsevier) 2:951-958
Page TL. 2009b. Circadian systems: Evolution. Encyclopedia of Neuroscience (Elsevier) 2:989-995
Passano LM, McCullough CB. 1962. The light response and the rhythmic potentials in Hydra. PNAS 48:1376-1382.
Passano LM, McCullough CB. 1963. Pacemaker hierarchies controlling the behaviour of hydras. Nature 199: 1174-1175.
Passano LM, McCullough CB. 1964. Co-ordinating systems and behaviour in Hydra. I. Pacemaker system of the periodic contractions. J. Exp. Biol. 41:643-664.
Passano LM, McCullough CB. 1965. Co-ordinating systems and behaviour in Hydra. II. The rhythmic potential system. J. Exp. Biol. 42:205-31.
Plachetzki DC, Degnan BM, Oakley TH. 2007. The origins of novel protein interactions during animal opsin evolution. PLoS ONE 2(10):e1054.
Preuss T, Budelmann BU. 1995. A dorsal light reflex in a squid. J. Exp. Biol. 198:1157-1159.
Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. 2000. A novel human opsin in the inner retina. J Neurosci. 20:600-605.
Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. 1998. Melanopsin: an opsin in melanophores, brain, and eye. PNAS 95:340-345.
Renninger GH, Chamberlain SC. 1993. Modulation of photoreceptor function in Limulus polyphemus by a central circadian clock. In: Sensory systems of arthropods (Wiese K, Gribakin FG, Popov AV, Renninger G, eds.) Birkhäuser, Basel pp. 307-316.
Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, Provencio I. 2008. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord 114:279-285.
Santillo S, Orlando P, De Petrocellis L, Cristino L, Guglielmotti V, Musio C. 2006. Evolving visual pigments: Hints from the opsin-based proteins in a phylogenetically old eyeless invertebrate. BioSystems 86:3-17.
Santillo S, Orlando P, De Petrocellis L, Musio C. 2009. Diurnal and circadian expression of genes encoding opsin-like photoreceptor proteins in Hydra, in preparation.
Sawyer SJ, Dowse HB, Shick M. 1994. Neurophysiological correlates of the behavioral response to light in the sea anemone Anthopleura elegantissima. Biol. Bull. 186:195-201.
Sgarbossa A, Checcucci G, Lenci F. 2002. Photoreception and photomovements of microorganisms, Photochem. Photobiol. Sci. 1:459-467.
Shiga S, Numata H. 2007. Neuroanatomical approaches to the study of insect photoperiodism. Photochem Photobiol. 83:76-86.
Simon TW, Edwards DH. 1990. Light-evoked walking in crayfish: behavioural and neuronal responses triggered by the caudal photoreceptor. J. Comp. Physiol. 166:745-755.
Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Pickard GE. 2003. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis. Neurosci. 6:601-610.
Stieve H, Nagy K. 1997. Phototransduction in Limulus photoreceptors and enigmas of the transduction mechanism in photoreceptor cells of invertebrates, In: Biophysics of photoreception: molecular and phototransductive events (Taddei-Ferretti C, ed.) World Scientific, Singapore pp. 134-159.
Suga H, Schmid V, Gehring WJ. 2008. Evolution and functional diversity of jellyfish opsins. Curr Biol 18:51-55.
Taddei-Ferretti C, Musio C. 1999. The neural net of Hydra and the modulation of its periodic activity. Lect Not Comp Sci 1606:123-137.
Taddei-Ferretti C, Musio C. 2000. Photobehaviour of Hydra and correlated mechanisms: a case of extraocular photosensitivity. J. Photochem. Photobiol. B: Biol. 55:88-101.
Taddei-Ferretti C, Di Maio V, Musio C, Cotugno A. 1992. Modulation of Hydra attenuata rhythmic activity. VI. Combined effects of background and pulse light wavelength. J. Photochem. Photobiol. B: Biol. 15:307-315.
Taddei-Ferretti C, Musio C, Santillo S, Cotugno A. 2004. The photobiology of Hydra's periodic activity. Hydrobiologia 530/531:129-134.
Terakita A, Tsukamoto H, Koyanagi M, Sugahara M, Yamashita T, Shichida Y. 2008. Expression and comparative characterization of Gq-coupled invertebrate visual pigments and melanopsin. J Neurochem. 105:883-90.
Terakita A, 2005. The opsins. Genome Biol. 6:213.
Truman JW. 1976. Extraretinal photoreception in insects. Photochem. Photobiol. 23:215-225.
Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. 2009. Melanopsin as a sleep modulator: Circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4-/- mice. PLoS Biol 7(6): e1000125.
Tu DC, Batten ML, Palczewski K, Van Gelder RN. 2004. Nonvisual photoreception in the chick iris. Science 306:129-131.
Vallone D, Lahiri K, Dickmeis T, Foulkes NS. 2007. Start the clock! Circadian rhythms and development. Dev. Dyn. 236:142-55.
Velarde RA, Sauer CD, Walden KK, Fahrbach SE, Robertson HM. 2005. Pteropsin: a vertebrate-like non-visual opsin expressed in the honey bee brain. Insect Biochem. Mol. Biol. 35:1367-1377.
Vigh B, Manzano MJ, Zádori A, Frank CL, Lukáts A, Röhlich P, Szél A, Dávid C. 2002. Nonvisual photoreceptors of the deep brain, pineal organs and retina. Histol. Histopathol. 17:555-90.
Warren EJ, Allen CN, Brown RL, Robinson DW. 2003. Light responses of retinal ganglion cells projecting to the circadian system. Eur. J. Neurosci. 17:1727-1735.
Wassermann GS. 1973. Unconditioned response to light in Limulus: mediation by lateral, median and ventral eye loci. Vision Res. 13:95-105.
Wiederhold ML, MacNichol Jr. EF, Bell AL. 1973. Photoreceptor spike responses in the hardshell Mercenaria mercenaria. J. Gen. Physiol. 61:24-55.
Wilkens LA. 1988. The crayfish caudal photoreceptor: advances and questions after the first half century. Comp. Biochem. Physiol. 91C:61-68.
Wolken JJ. 1988. Photobehavior of marine invertebrates: Extraocular photoreception. Comp Biochem Physiol C. 91:145-9.
Wolken JJ. 1995. Light detectors, photoreceptors, and imaging systems in nature. Oxford University Press, Oxford.
Wolken JJ, Mogus MA. 1981. Extraocular photoreception. In: Photochemical and Photobiological Reviews, Vol. 6 (Smith KC, ed.) Plenum, New York pp. 181-199.
Yoshida M. 1979. Extraocular photoreception. In: Handbook of Sensory Physiology, Vol. VII/6A, Comparative physiology and evolution of vision in invertebrates. (Autrum H, ed.) Springer, New York pp. 582-640.
8/17/09