PHOTOMOVEMENT of CILIATES
Francesco Lenci, Giovanni Checcucci, Antonella Sgarbossa
CNR Istituto Biofisica
Via G. Moruzzi 1, 56100 Pisa, ITALY
francesco.lenci@pi.ibf.cnr.it
giovanni.checcucci@pi.ibf.cnr.it
antonella.sgarbossa@pi.ibf.cnr.it
Ciliates, generally considered among the most evolved and complex protozoa, are able to perceive and react to a wide variety of environmental stimuli (such as chemical, mechanical, thermal, gravitational, photic...), modifying the beating pattern of their cilia, the short hair-like organelles that cover their whole body, and, consequently, their movement. This capability to perceive and react to different stimuli allows ciliates to bring into being complex and variable adaptive motile responses to wide-ranging and continuously changing external conditions, thus integrating their "internal needs" (such as metabolism and cell cycle) with the features of the environment they live in.
Even though ciliated protozoa do not harvest and convert light energy directly for their metabolism, some of them are able to perceive and react also to photic stimuli. For ciliates, light can be an environmental cue to gather into habitats that can be unfavorable for their predators and propitious for their prey and, in general, for food. Some colored ciliates, moreover, contain endogenous photosensitizer(s), generally used as defensive pigment(s) against predators, and even relatively dim light can cause severe damage, and their capability of escaping lighted spots is directly linked to their survival.
These organisms are provided with sophisticated molecular machineries allowing them to exploit a large variety of biophysical/biochemical pathways, the identification and the characterization of the molecular mechanisms underlying the sensory transduction chain being not trivial at all. As a matter of fact, in most of them, even the chemical nature of the molecule acting as light detector is still in doubt, and the molecular mechanisms by which the light signals are transduced to the ciliary apparatus are undetermined (Table 1).
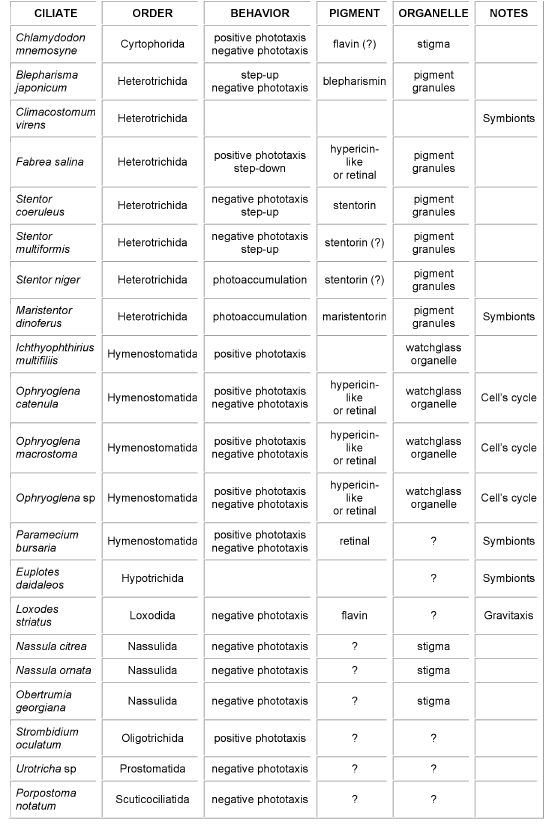
Table 1.Schematic summary of ciliates showing photomotile responses, type of photobehavior, and where known, candidate receptor pigment and organelle. In the last column (Notes), "symbionts" is a warning that a symbiotic unicell (usually algae) might be the "real" photoresponding organism, while "Cell's cycle" indicates that the organism photosensitivity depends upon the phase of its life cycle.
Stentor coeruleus and Blepharisma japonicum are probably the most deeply investigated ciliates (Sobierajska et al., 2006), and this is the reason why in the following we will focus our attention mainly on these two cases. It is worthwhile to stress, however, that recently another heterotrich ciliate, Maristentor dinoferus, has been shown to react to light stimuli (Mukherjee et al., 2006). Its photosensing chromophore is structurally similar to those of Stentor and Blepharisma, the major difference being that it bears no aromatic hydrogens.
In S. coeruleus and B. japonicum (Figure 1), hypericin-like chromophores, stentorin and blepharismin, respectively, have been demonstrated to trigger the photomotile reactions, and their structural formulas have been determined in detail. Despite this knowledge, the chromophore molecular pocket and the photo-induced primary reactions have not been definitively clarified, and the "dark steps" of the sensory transduction chain are still largely black boxes.

Figure 1. Blepharisma japonicum (scanning electron micrograph).
Photobehavioral Responses
A sudden increase in light intensity causes S. coeruleus to stop swimming, reverse the direction of its ciliary beating, tumble until adaptation occurs, and forward movement is resumed. When the cell crosses a dark-light border, the final outcome of these maneuvers is a casual change in the direction of swimming, which eventually allows the cell to escape from the lighted region.
In addition to this step-up photophobic reaction, S. coeruleus is also able to detect the direction of the incident light, and to swim away from the light source (negative phototaxis, see basic module on Photomovement) (Song et al., 1980b) (Figure 2).
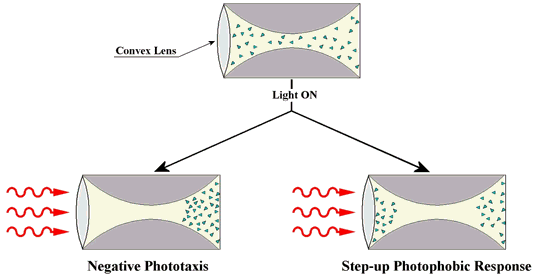
Figure 2. Motile responses of a cell population to a directional light stimulus obtained by means of a converging lens. The chambers, shown above, have exactly the same shape as the light field. The two chambers demonstrate phototaxis and a photophobic response.
Similarly to the case of S. coeruleus, B. japonicum has been shown to accumulate in shaded regions (Giese, 1973, 1981). The step-up photophobic reaction of B. japonicum, closely resembling the response exhibited by S. coeruleus, consists of a stop of forward movement, followed by backward swimming along a bent trajectory, so that the cell's longitudinal axis changes its direction (see module on Basic Photomovement). Under continuous illumination the light-avoiding maneuver is repeated until the cell adapts to the new illumination conditions. The step-up photophobic reaction of B. japonicum occurs with a time lag with respect to the stimulus application, which decreases with increasing photon flux density, and depends on the actinic wavelength (Ghetti, 1991).
In B. japonicum, it has also been shown that not only native blepharismin elicits step-up photophobic responses, but also oxyblepharismin, which is the product of an intracellular photooxydation of the native chromophore (Spitzner et al., 1999). In fact, if B. japonicum is exposed to relatively strong light, in the presence of oxygen, blepharismin acts as a strong photosensitiser, and readily causes its death. (Giese, 1973, 1981; Ghetti et al., 1992), but under relatively low intensity irradiation (below 10 W/m2), the red form of blepharismin progressively converts into a grey-blue form, oxyblepharismin, apparently neither toxic nor phototoxic for the cell (Giese, 1981; Ghetti, 1991) (Figure 3).
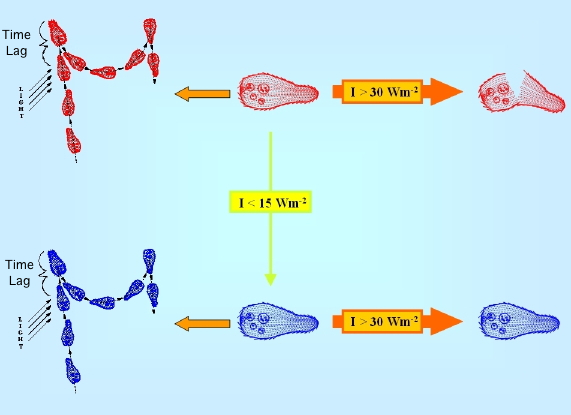
Figure 3. Schematic drawing of the photokilling vs. photooxydation processes in B. japonicum. Blepharismin-containing red cells are converted to oxyblepharismin-containing blue cells, because of chromophore photooxidation under low white light intensity illumination (I<15 Wm2). Under high white light intensity irradiation (I>30 Wm2), red cells are much more easily photokilled (broken cells) through a photodynamic reaction sensitized by the pigment than blue ones.
Several action spectra have shown that stentorin is the photosensing chromophore of S. coeruleus, and blepharismin and its photo-oxidized form, oxyblepharismin, are those of B. japonicum (Figures 4 and 5).
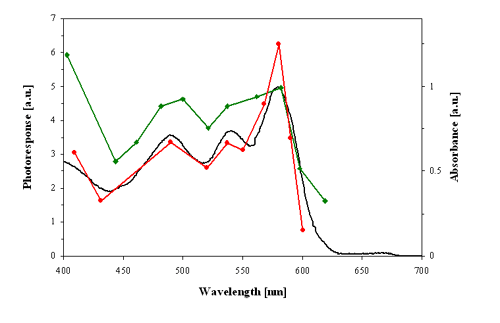
Figure 4. Action spectra for the step-up photophobic responses (strength of the photoresponse in arbitrary units, a.u.) of red cells of Blepharisma japonicum. Red line: action spectrum determined by Checcucci et al. (1993). Green line: action spectrum determined by Matsuoka et al. (1992). Black line: optical absorption spectrum of blepharismin (red pigment).
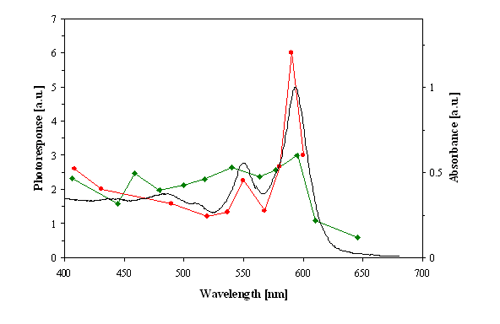
Figure 5. Action spectra for the step-up photophobic responses (strength of the photoresponse in arbitrary units, a.u.) of blue cells of Blepharisma japonicum. Red line: action spectrum determined by Checcucci et al. (1993). Green line: action spectrum determined by Matsuoka et al. (1992). Black line: optical absorption spectrum of oxyblepharismin (blue pigment).
These pigments are mainly localized in granules about 500 nm in diameter, are membrane-limited and arranged in strings parallel to the ciliary rows spread all over the cellular body. A honeycomb-like structure, made up of a folded membrane, has also been suggested to be contained in the pigment granules (Matsuoka et al., 1994, 2000a) (Figures 6 and 7). Recently, it has been shown that in several ciliates the candidate photoreceptor pigments are localized also in the cilia (see module on Confocal Microscopy of Ciliates).
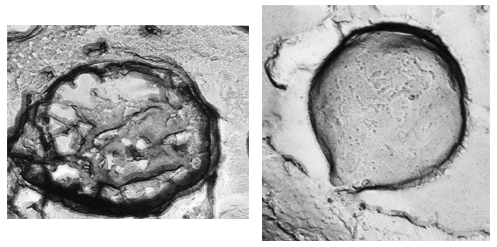
Figure 6. Blepharisma japonicum granules (freeze fracture; Matsuoka et al., 1994). Left: inside structure of a granule. Right: external surface of a granule.
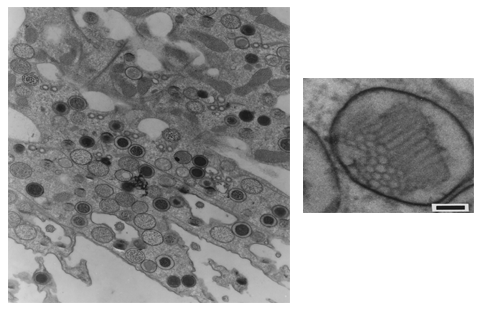
Figure 7. Blepharisma japonicum granules (transmission electron micrograph of sliced cells; Matsuoka et al., 1994). Right: cell section containing several granules (low magnification). Left: inner structure of a granule (high magnification).
For B. japonicum and S. coeruleus, immunoblotting of isolated cell membrane fractions seems to suggest that both these ciliates contain a rhodopsin-like protein within the cell membrane, which may function as a receptor molecule in the sensory transduction pathway mediating motile photoresponses (Fabczack et al., 2008). As in any other case, however, the presence of any pigment/chromophore in a unicell is not enough to conclude that that pigment is THE photoreceptor. Unambiguous evidence is needed to show that the pigment triggers the photobehaviour.
Photoreceptor Pigments and Primary Reactions
In the landscape of the chromophores of photoreceptors for photomotile responses of microorganisms (retinal, 4-hydroxycinnamic acid, flavins, pterins; see module on Basic Photomovement), the hypericin analogs stentorin, blepharismin and oxyblepharismin, maristentorin, and possibly fabrein, constitute a new unique class of photopigments (Lobban et al., 2007). The blepharismin structure (Figure 8) is particularly unique among the hypericin-like compounds, because of its bridge carbon, which disrupts conjugation and accounts for its distinct absorption spectrum. Unlike rhodopsins and Photoactive Yellow Protein (PYP), neither stentorin nor blepharismins exhibit a photochemical transformation cycle that can be readily detected by spectrophotometry.
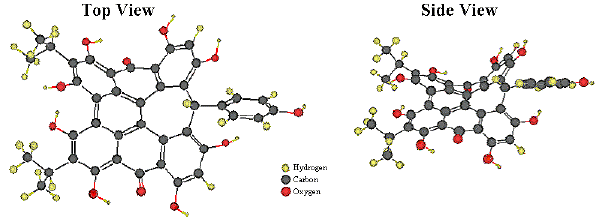
Figure 8. Ball and Stick Models of Blepharismin.
As these dianthronic molecules (molecules with two anthroquinione groups) are efficient photosensitisers, able to yield singlet oxygen or other reactive oxygen species (ROS) from their first excited triplet state (see Modules on Photosensitization), a reasonable hypothesis could be that, inside the cell, ROS are detected as a signal and transduced by a chemoreceptor (photochemosensing), similarly to what was observed in the ciliate Loxodes striatus (Finlay and Fenchel,1986). However, this hypothesis was ruled out by the lack of any significant effect on photomotile responses of crocetin, a singlet oxygen quencher, and of DMPO (5,5-Di-Methyl-1-Pyrroline-1-Oxide), a ROS scavenger (Checcucci et al., 1991; Kida et al., 2000).
Much experimental evidence points to the occurrence in these protozoa of a light-induced transient intracellular pH decrease (Song, 1983; Fabczak et al., 1993). According to these observations, a light-driven proton release from the first excited singlet state of the chromophores was suggested to be the primary step of the transduction chain.
Proton release from the first excited triplet state is at present an untested hypothesis that, in our opinion, deserves deeper examination. The hypothesis of a light-driven proton translocation process as a primary step in the sensory process is, however, in reasonably good agreement with Matsuoka's findings that in an aqueous solution of blepharismin, a pH decrease of 0.04 units is observed following two minutes irradiation with white light, 1000 W m2 (Matsuoka et al., 1992).
EPR (electron paramagnetic resonance) and fluorescence quenching experiments seem to indicate an electron transfer from the excited singlet state of the chromophore to a suitable physiological acceptor molecule (e.g., apoprotein) as the possible primary photoprocess in the photosensory transduction chain of these ciliates (Wells et al., 1997; Angelini et al., 1998; Plaza et al., 2005).
In the case of Maristentor dinoferus, it is remarkable that while the candidate receptor pigments are structurally similar to those of Stentor and Blepharisma, in the former they give rise to an abrupt photophobic response, and in the latter to a nonabrupt photophilic response. Clearly the immediate environment of the chromophore, e.g., the protein to which it is attached, modulates its photoinduced properties (Mukherjee et al., 2006).
Two distinct forms of stentorin chromoproteins have been chromatographically isolated, stentorin I and II. The weakly fluorescent stentorin II contains two subunits, stentorin II-A and II-B, in which the chromophore is covalently bound to a 50-kDa protein (Kim et al., 1990; Song et al., 1990). This latter complex is suggested to be the photosensor of S. coeruleus, but up to now the protein structure is still unknown.
Even more uncertain are the molecular properties of the blepharismin-binding protein(s), and the nature of the binding itself. Different research groups, in fact, had proposed hardly reconcilable interpretations of their biochemical data. According to the resulting puzzling picture, the chromophore might be non-covalently bound either to an apoprotein with a 38 kDa molecular mass (Gioffre et al., 1993) or to a 200 kDa single polypeptide chain (Matsuoka et al., 1993, 1994, 1997). Finally, immunological and biochemical evidence suggests that a specific carrier protein for the blepharismin chromophore might not exist at all (Podesta et al., 2000). Recent findings comparing spectroscopic properties and the singlet oxygen production rate of the free pigment and of the putative pigment-protein complex, seem to support the 200 kDa hypothesis, and suggest that at least oxyblepharismin is buried in the protein in a site poorly accessible to oxygen (Checcucci et al., 2001; Plaza et al., 2005).
As mentioned above, blue cells still display the same phobic response as red cells, but a stable chromophore-protein complex can be extracted from them. This complex is made of a non-soluble 200-kDa protein, non-covalently bound to the chromophore identified as responsible for the photophobic response, namely oxyblepharismin (OxyBP). The primary structure of OxyBlepharismn bInding Protein (OBIP) is still unknown, but comparative subpicosecond transient-absorption studies of OBIP and OxyBP (Plaza et., 2005, 2007; Mahet et al., 2007) have shown that they exhibit a quite different and specific photoinduced behavior. It has also been found to have a close similarity with the spectrum of the OxyBP radical cation, an analogy pointing toward electron transfer as the primary photoinduced process in OBIP, in agreement with the previous proposal of Angelini et al. (1998).
Photosensory Transduction Chain: Dark Steps
As mentioned above, photobehavioral studies (carried out by varying extracellular pH and in the presence of suitable protonophores or ammonium chloride) point to a photoinduced intracellular pH decrease as a key step for inducing the photomotile responses of B. japonicum and S. coeruleus. Furthermore, electrophysiological studies and experiments on the effect of calcium channel blockers and calcium ionophores on photomotile responses clearly indicate that calcium influx across the cellular membrane is responsible for ciliary arrest and beating reversal (stop response and backward swimming) (Wood, 2001). On the basis of these data, an initial hypothesis was that, following light stimulation, an intracellular increase of proton concentration could lead to the opening of calcium channels, indirectly, by depolarizing the membrane, or, directly, by altering the conductance of specific calcium channels.
More recent results enrich and complicate this simple model, suggesting the involvement of amplification and transduction steps similar to those operating in the visual process of metazoans. The fact that, in B. japonicum and S. coeruleus the light-induced ciliary arrest occurs with a delay up to 1 second (significantly long in comparison with the milliseconds lag-time of mechanoresponses in the same organisms) has been considered indicative of specific time-limiting biochemical processes operating in the phototransduction chain (Fabczak, 2000).
Modulators of G-Proteins have been tested on the photobehaviour of B. japonicum and S. coeruleus, confirming their possible involvement in the photic signal processing of these ciliates (Fabczak et al., 1999; Matsuoka et al., 2000b; Fabczak, 2000; Walerczyk and Fabczak, 2001).
Recent studies of the in vivo colocalization and interaction of phosducin (Pdc) with the subunits of G-protein in the ciliate. Blepharisma japonicum, provide additional detailed characterization of the functional properties of the ciliate Pdc, and point to a key functional role of Pdc, in Blepharisma (Sobierajska et al., 2007).
Concluding Remarks
Perylenequinone-type chromophores, as light-detecting pigments, have only been found, thus far, in some ciliates. It has been suggested that in these chromophores, proton and/or electron light-induced transfer could trigger the phototransduction chain. Very recently, however, for B. japonicum and S. coeruleus, on the basis of immunoblotting of isolated cell membrane fractions, it has been suggested that both of these ciliates contain a rhodopsin-like protein within the cell membrane, which may function as a receptor molecule in the sensory transduction pathway (Fabczack et al., 2008). As in any other case, however, the presence of any pigment/chromophore in a unicell is not enough to conclude that it is THE photoreceptor. Unambiguous evidence is needed to prove that the pigment does trigger the photobehaviour.
In conclusion, at present no unequivocal experimental evidence is available to draw final conclusions on the nature of the photoreceptor pigment for photomovement, even if the blepharismin hypothesis seems strongly supported by a number of experimental findings. The isolation and the characterization of the blepharismin-binding protein(s) could provide excellent hints to clarify this question.
References
Angelini N., A. Quaranta, G. Checcucci, P.-S. Song and F. Lenci (1998) Electron transfer fluorescence quenching of Blepharisma japonicum photoreceptor pigments. Photochem. Photobiol. 68, 864-868.
Checcucci, G, G. Damato, F. Ghetti and F. Lenci (1993) Action spectra of the photophobic response of the blue and red forms of Blepharisma japonicum. Photochem. Photobiol. 57, 686-689.
Checcucci G., F. Lenci, F. Ghetti and P.-S. Song (1991) A videomicroscopic study of the effect of a singlet oxygen quencher on Blepharisma japonicum photobehavior. J. Photochem. Photobiol., B: Biol., 11, 49-55.
Checcucci G., R. K. Shoemaker, E. Bini, R. Cerny, N. Tao, J.-S. Hyon, D. Gioffre, F. Ghetti, F. Lenci and P.-S. Song (1997) Chemical structure of blepharismin, the photosensor pigment for Blepharisma japonicum. J. Am. Chem. Soc.,119, 5762-5763.
Checcucci G., Y. Takada and T. Matsuoka (2001) Studies on the photoreceptor pigment-protein complex of the ciliate Blepharisma japonicum. Mem Fac. Sci. Kochi Univ., Ser. D (Biol), 22, 39-44.
Fabczak H. (2000) Protozoa as model system for studies of sensory light transduction: photophobic response in the ciliate Stentor and Blepharisma. Acta Protozool., 39, 171-181.
Fabczak H., S. Fabczak, P.-S. Song, G. Checcucci, F. Ghetti and F. Lenci (1993) Photosensory transduction in ciliates. Role of intracellular pH and comparison between Stentor coeruleus and Blepharisma japonicum. J. Photochem. Photobiol., B: Biol., 21, 47-52.
Fabczak, H., Sobierajska, K., and Fabczak, S. (2008) A rhodopsin immunoanalog in the related photosensitive protozoans Blepharisma japonicum and Stentor coeruleus. Photochem. Photobiol. Sci, 7, 1041-1045.
Fabczak H., M. Walerczyk, B. Groszynskaand and S. Fabczak (1999) Light induces inositol trisphosphate elevation in Blepharisma japonicum. Photochem. Photobiol., 69, 254-258.
Finlay B.J. and T. Fenchel (1986) Photosensitivity in the ciliated protozoon Loxodes: pigment granules, absorption and action spectra, blue light perception, and ecological significance. J. Protozool., 33, 534-542.
Ghetti F. (1991) Photoreception and photomovements in Blepharisma japonicum. In Biophysics of Photoreceptors and Photomovemets in Microorganisms, (F. Lenci, G. Colombetti, F. Ghetti and P.-S. Song,. Eds.), Plenum Press, New York, pp. 257-265.
Ghetti, F., Checcucci, G., and Lenci, F.: Photosensitized reactions as primary molecular events in photomovements of microorganisms (1992) J. Photochem. Photobiol. B: Biol., 15 185-198.
Giese A. C. (1973) Blepharisma. The biology of a light-sensitive protozoan. Stanford University press, Stanford, California.
Giese A. C. (1981) The photobiology of Blepharisma. Photochem. Photobiol. Rev., 6, 229-255.
Kida A., Y. Takada, H. Kotsuki, D. Tokumori, G. Checcucci and T. Matsuoka (2000) Primary stages in photosignal transduction leading to step-up photophobic response in the unicellular eukaryote Blepharisma japonicum. Microbios, 106, 189-201.
Kim I.-H., J. S. Rhee, J. W. Huh, S. Florell, B. Faure, K. W. Lee, M. Kahsai, P.-S. Song, N. Tamai, T. Yamazaki and I. Yamazaki (1990) Structure and function of the photoreceptor stentorins in Stentor coeruleus. I. Partial characterization of the photoreceptor organelle and stentorins. Biochim. Biophys. Acta, 1040, 43-57.
Kraml M. and W. Marwan (1983) Photomovement response of the heterotrichous ciliate Blepharisma japonicum. Photochem. Photobiol. 37, 313-319.
Lenci F., F. Ghetti and P.-S. Song (2001) Photomovements of ciliates. . In Photomovements (D.-P Hader and M. Lebert, Eds.), Comprehensive Series in Photosciences (D.-P. Hader and G. Jori, Eds.), Vol 1, Elsevier, Amsterdam, 475-503.
Lobban, C. S., Hallam, S. J., Mujherjee, P., and Petrich, J. W. (2007) Photophysics and Multifunctionality of Hypericin-Like Pigments in Heterotrich Ciliates: A Phylogenetic Perspective. Photochem. Photobiol., 83, 1074-1094.
Mahet, M., Plaza, P., Martin, M. M., Checcucci, G. and Lenci, F (2007) Primary photoprocesses in oxyblepharismin interacting with its native protein partner. J. Photochem. Photobiol. A: Chemistry, 185, 345-353.
Matsuoka, T., S. Matsuoka, Y. Yamaoka, T. Kuriu, Y. Watanabe, M. Takayanagi, Y. Kato and K. Taneda (1992) Action spectra for step-up photophobic response in Blepharisma. J. Protozool. 39, 498-502.
Matsuoka T., Y. Murakami, T. Furukohri, M. Ishida and K. Taneda (1992) Photoreceptor pigment in Blepharisma: H+ release from red pigment. Photochem. Photobiol., 56, 399-402.
Matsuoka T., Y. Murakami and Y. Kato (1993) Isolation of blepharismin-binding 200 kDa protein responsible for behavior in Blepharisma. Photochem. Photobiol., 57, 1042-1047.
Matsuoka T., T. Tsude, M. Ishida, Y. Kato, M. Takayanagi, T. Fujino, and S. Mizuta (1994) Presumed photoreceptor protein and ultrastructure of the photoreceptor organelle in the ciliated protozoa, Blepharisma. Photochem. Photobiol. 60, 598-604.
Matsuoka T., M. Sato, M. Maeda, H. Naoki, T. Tanaka and H. Kotsuki (1997) Localization of blepharismin photosensors and identification of a photoreceptor complex mediating the step-up photophobic response of the unicellular organism, Blepharisma. Photochem. Photobiol., 65, 915-921.
Matsuoka T., D. Tokumori, H. Kotsuki, M. Ishida, M. Matsushita, S. Kimura, T Itoh and G. Checcucci (2000a) Analyses of structure of photoreceptor organelle and blepharismin-associated protein in unicellular eukaryote Blepharisma. Photochem. Photobiol., 72, 709-713.
Matsuoka T., N. Moriyama, A. Kida, K. Okuda, T. Suzuki and H. Kotsuki (2000b) Immunochemical analysis of a photoreceptor protein using anti-IP3 receptor antibody in the unicellular organism, Blepharisma. J. Photochem. Photobiol., B: Biol., 54, 131-135.
Mukherjee, P., Fulton, D. B., Halder, M., Han, X., Armstrong, D. W., Petrich, J. W. and Lobban, C. S. (2006) Maristentorin, a novel pigment from the positively phototactic marine ciliate Maristentor dinoferus, is structurally related to hypericin and stentorin. J. Phys. Chem. B, 110, 6359-6364.
Plaza, P., Mahet, M., Martin, M. M., Angelini, N., Malatesta, M., Checcucci, G. and Lenci, F. (2005) Spectroscopic study of the chromophore-protein association and primary photoinduced events in the photoreceptor of Blepharisma japonicum, Phochem. Photobiol. Sci.,4, 754-761.
Plaza, P., Mahet, M., Martin, M. M., Checcucci, G. and Lenci, F. (2007) Target analysis of primary processes involved in the Oxyblepharismin-Binding Protein J. Phys. Chem. B, 111, 690-696.
Podesta A., D. Gioffre, T. Grossi and G. Montagnoli (2000) Immunological and biochemical evidence that blepharismin is not a prosthetic group. Photochem. Photobiol., 71, 669-673.
Sobierajska, K., Fabczak, H., and Fabczak, S. (2006) Photosensory transduction in unicellular eukaryotes: A comparison between related ciliates Blepharisma japonicum and Stentor coeruleus and photoreceptor cells of higher organisms J. Photochem. Photobiol. B: Biology 83, 163-171.
Sobierajska, K., Fabczak, H., and Fabczak, S. (2007) Phosducin interacts with the G-protein beta-gamma-dimer of ciliate protozoan Blepharisma japonicum upon illumination. J. Exp. Biol. 210, 4213-4223.
Song P.-S. (1983) Protozoan and related photoreceptors: molecular aspects. Annu. Rev. Biophys. Bioeng., 12, 35-68.
Song P.-S., D.-P. Hader, K. L. Poff (1980) Phototactic orientation by the ciliate Stentor coeruleus. Photochem. Photobiol., 32, 781-786.
Song P.-S., I.-H. Kim, S. Florell., N. Tamai, T. Yamazaki and I. Yamazaki (1990) Structure and function of the photoreceptor stentorins in Stentor coeruleus. II. Primary photoprocess and picosecond time-resolved fluorescence. Biochim. Biophys. Acta, 1040, 58-65.
Spitzner, D., Hofle, G., Klein, I., Pohlan, S., Ammermann, D., Jaenicke, L.(1998) On the structure of oxyblepharismin and its formation from blepharismin. Tetrahedron Letters 39, 4003-4006.
Walerczyk M. and S. Fabczak (2001) Additional evidence for the cyclic GMP signalling pathway resulting in the photophobic behavior of Stentor coeruleus. Photochem. Photobiol, 74, 829-836.
Wells T.A., A. Losi, R. Dai, M. Anderson, J. Redepenning, P. Scott, S.-M. Park, J. Golbeck, and P.-S. Song (1997) Electron transfer quenching and photoinduced EPR of hypericin and the ciliate photoreceptor stentorin. J. Phys. Chem. 101, 366-372.
Wood D. C. (2001) Electrophysiology and light responses in Stentor and Blepharisma. In Photomovements (D.-P Hader and M. Lebert, Eds.), Comprehensive Series in Photosciences (D.-P. Hader and G. Jori, Eds.), Vol 1, pp. 505-518.
9/29/08