ANALYTICAL APPLICATIONS OF LUMINOUS BACTERIA ENZYMES
Valentina Kratasyuk1,2, Elena Esimbekova2,1
1Institute of Fundamental Biology and Biotechnology, Siberian Federal University, Svobodnyi av. 79, Krasnoyarsk 660041, Russia
valkrat@mail.ru
2Institute of Biophysics SB RAS, Akademgorodok 50/50
Krasnoyarsk 660036, Russia
esimbekova@yandex.ru
Introduction
Historically, the application of bacterial bioluminescence in toxicology began with the use of a culture of bioluminescent bacteria for ecological monitoring, and this method is still widely used (Girotti et al., 2008; Fernández-Piñas et al., 2014; Xu et al., 2014). It was demonstrated that environmental pollution could be monitored by comparing the light emission intensity of a luminous bacteria culture as a control, with a culture after sample addition. As opposed to other test organisms, such as paramecia, algae, crustaceans, and so on, the bioluminescent assay is faster (typically < 30 min). However, the failure to maintain a stable state of a bacterial culture during measurement and storage results in low accuracy of measurement, a clear disadvantage of this method. The bacteria react to the appearance of toxic substances either by decreasing or by increasing the luminous intensity, often leading to an ambiguous interpretation of results.
To overcome these difficulties, it was suggested to use the enzymes purified from bioluminescent bacteria, NAD(P)H:FMN-oxidoreductase and bacterial luciferase, in soluble and immobilized forms (Kratasyuk, 1990; Esimbekova et al., 2013). Since 1990, this bioluminescent enzymatic toxicity assay has been highly developed (Kratasyuk, 1990), and nowadays it is actively used in ecology, medicine, agriculture, and other areas (Esimbekova et al., 2014; Roda et al., 2009).
Bioluminescent Enzymatic Assays in Ecotoxicology
The bacterial coupled enzyme system: NAD(P)H:FMN-oxidoreductase + luciferase (Red + Luc) involves two reactions:
FMNH2 + RCHO + O2 → FMN + RCOOH + H2O + hν (1)
NAD(P)H:FMN-oxidoreductase (Red)
NAD(P)H + FMN + H+ → NAD(P)+ + FMNH2 (2)
In Reaction 1, bacterial luciferase (Luc) catalyzes the oxidation of a long-chain aliphatic aldehyde (RCHO) involving reduced flavin mononucleotide. One of the products of this reaction is a quantum of light (hν) in the blue-green spectrum. To provide luciferase with reduced flavin mononucleotide, the luciferase reaction is coupled with the reaction catalyzed by NAD(P)H:FMN-oxidoreductase (Red) (Reaction 2) (Shimomura, 2006; Lin and Meighen, 2009). Application of bioluminescent enzymatic toxicity assays is justified by the fact that Red, as a part of these enzymatic assays, is present in all living organisms, leading to good correlation between the effect of toxic substances on living organisms, and that of the coupled enzyme system from the luminous bacteria. The main principle of the bioluminescent enzymatic toxicity assay (the “luciferase biotest”) is the inhibition of Red and/or Luc activities by the toxic components of analyzed samples (Figure 1).
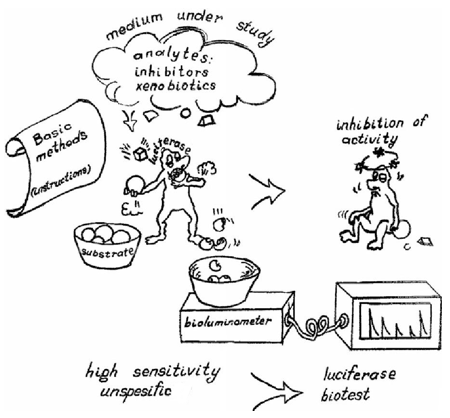
Figure 1. Cartoon illustrating the luciferase biotest.
A classification of inhibitors, according to the mechanism of their influence on the luciferase biotest, was proposed by Kudryasheva (2006). There are four possible ways in which exogenous compounds act on the bioluminescence: 1) influence on energy transport processes, 2) influence on hydrogen transport processes, 3) influence on electron transfer processes in bioluminescent enzymatic reactions, and 4) interaction of pollutants with the enzymes of the coupled enzyme system Red + Luc. Knowing the mechanisms, it is possible to predict the results and change the sensitivity of assays to certain pollutant groups.
The bioluminescent enzymatic toxicity assay can be carried out using different schemes (Figure 2). The first scheme places a cuvette with all the necessary components of the bacterial coupled enzyme system (enzymes, their substrates and buffer solution) into a bioluminometer, register the maximum steady light emission intensity Ic (control), then add the sample or pollutant solution into the cuvette, and again register the maximum light emission intensity Isam (Figure 2A). This approach is the quickest, and has demonstrated a good repeatability of results.

Figure 2. (A) Bioluminescent assay scheme; (B) Modified scheme of bioluminescent assay. The Light is bioluminescence intensity in relative units; Iс and Isam are maximum values of bioluminescence intensity in the presence of control or analyzed sample respectively; Tmax is the time when the coupled enzyme system reached the luminescence maximum; P is a time when the bioluminescent signal is absent due to an effect of redox active compounds in a sample.
When analyzing toxicity of water samples, the luciferase index (LI) or toxicity coefficient (TC) are calculated according to the formulas:
TC = [(Ic – Isam)/ Ic] ·100 %
TC = 100 - LI
LI and TC are the residual luminescence and the degree of inhibition, respectively, of the bacterial coupled enzyme system Red + Luc in the presence of the analyzed sample. The criterion of toxicity is a 50 % decrease in the maximum of light emission for the bacterial coupled enzyme system Red + Luc after the analyzed sample is added. To compare the toxicity of individual substances, values used for TC are the 50% and 20% loss of luminescence for the coupled enzyme system Red + Luc.
The second scheme (Figure 2B) involves comparing the luminescence level of the control sample (usually distilled water or buffer solution) and the analyzed sample in different cuvettes. This approach makes possible a higher sensitivity of the assays to the toxic substances. In addition to the 50% and 20% criteria, it is also possible to use one more parameter - the time when the coupled enzyme system reached the luminescence maximum (Tmax ; Figure 2B). An induction period (P; Figure 2B) can be observed in presence of redox active compounds when the bioluminescent signal appears after some period of its absence. It is due to thr competitive reduction of the compounds and FMN by NADH in reaction 2 (Kudryasheva, 2006).
The bioluminescent enzymatic toxicity assay was successfully used for the analysis of samples from aquatic environments (Kratasyuk et al., 2001; Kratasyuk et al., 1999; Vetrova et al., 2002) as well as air and soil pollutants (Rimatskaya et al., 2012, 2014).
A new trend in using the bioluminescent enzymatic toxicity assay is the assessment of detoxification of pollutant solutions by water-soluble humic substances (HS) This method is based on the quantitative determination of the antioxidant activity of HS. There have been a few studies that promote application of the methodology to monitor toxicity of pollutants of oxidative nature in environmental and waste waters during remediation procedures (Tarasova et al., 2015). The bioluminescent enzymatic toxicity assays were also applied to monitor changes in the toxicity of homologous quinones with different redox characteristics under exposure to HS. Toxicities of general and oxidative types were evaluated using bioluminescent kinetic parameters—bioluminescence intensity and Tmax , respectively. Antioxidant activity of HS was attributed to their ability to decrease both general and oxidative toxicities. To characterize changes in general toxicity (GT) under the action of HS, detoxification coefficients, DGT, were calculated according to the formula:
where LIOX and LIHS+Ox are values of the residual luminescence in the presence of oxidizer and in the presence both oxidizer and HS, respectively. DGT>1 and DGT<1 indicate the decrease and increase of general toxicity of a solution, respectively.
To characterize changes of oxidative toxicity (OxT) under the action of HS, the Tmax in the presence both oxidizer and HS and in the presence the only oxidizer ((Tmax (HS+Ox) and (Tmax (Ox), respectively) were compared; detoxification coefficient, DOxT, was calculated as:
DOxT>1 and DOxT<1 indicate the decrease and increase of oxidative toxicity of the solution, respectively. Dependency of DGT and DOxT on HS concentration and time of preliminary incubation of the oxidizers with HS were demonstrated. The optimal conditions for detoxification of the oxidizers were > 20-min incubation time and from 50 µM to 0.2 mM of HS concentration.
The Set of Bioluminescent Enzymatic Toxicity Assays
The bioluminescent enzymatic toxicity assay provides an approach to the problem of the complex evaluation of environmental toxicity. It is well known that to estimate environmental toxicity broadly it is necessary to use a battery of bioassays. Usually the toxicity varies depending on different levels of organisms such as cells, organs, organisms and whole ecosystems. Due to the coupling with bacterial luciferase, it is possible to design novel enzymatic bioassays in toxicology, and combine them into a set to provide toxicity monitoring at the enzymatic level (Kratasyuk and Gitelson, 1987).
The set includes enzymes of different classes, or key enzymes of metabolic processes in living organisms. The bacterial luciferase may be the terminal enzyme in coupling chains for more than 100 enzymes including lactate dehydrogenase, trypsin, glucose-6-phosphate dehydrogenase, and others, making it possible to measure the enzyme activities according to the light emission intensity. To develop the set of bioluminescent enzymatic toxicity bioassays, different enzyme interaction mechanisms were suggested (Figure 3). For example, in research by Kratasyuk et al. (2001) to estimate the toxicity of water samples, two enzymes were chosen: alcohol dehydrogenase (ADH) and trypsin, because they belong to different classes (oxidoreductases and hydrolases), and secondly, because they interact differently with bacterial luciferase, providing sensitivity to the different toxic substances (Kudryasheva et al., 1999, 2003).
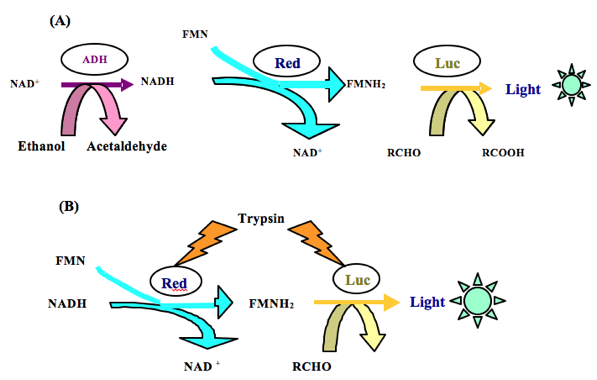
Figure 3. Examples of coupling of the enzymatic reactions. (A) The sequence of enzymes in the triple enzyme system: ADH + Red + Luc (Petushkov et al., 1987); (B) Interaction of enzymes in the triple enzyme system: trypsin + Red + Luc (Njus et al., 1974).
Moreover, it became possible to regulate the sensitivity of enzymatic toxicity assays. For example, it was shown that the sensitivity of enzymatic assays to the toxic substances may be increased by extending the coupling chain of enzymatic reactions (Figure 4).

Figure 4. Dependence of induction period (P) on the concentration (C) of 1,4-benzoquinone in the reaction mixture, (A) For the coupled enzyme system Red + Luc, and (B) For the triple enzyme system ADH + Red + Luc. Increasing the number of links in the coupling chain of enzymatic reactions results in an increase of sensitivity to benzoquinone from 1 µM to 0.01 µM (Kudryasheva et al., 1999).
The set of bioluminescent enzymatic toxicity assays was used for monitoring natural and laboratory aquatic ecosystems (Kratasyuk et al., 2001), and for studying the seasonal dynamics of zooplankton non-consumptive mortality (Dubovskaya et al., 2002), as well as for toxicity analysis of pesticides (Vetrova et al., 2007), and safety assessment of the natural polymers polyhydroxyalkanoates (Shishatskaya et al., 2002).
Other fields of Application of Bioluminescent Enzymatic Toxicity Assays
Bioluminescent enzymatic toxicity assays are used not only in ecology but also in other sectors, such as agriculture, the food industry and medicine.
The most frequently used method of assessing bacterial contamination is based on the ATP-dependent firefly luciferase. However, the enzymatic bioluminescent bacterial systems can also be applied for food product quality. For example, methods for detecting living bacterial cells were developed using luminous bacteria enzymes (Mei et al., 2009; Peng et al., 2014), and also for the determination of L-and D-lactate in beer (Girotti et al., 2000). The effects of the mycotoxins produced by Fusarium fungi on the coupled enzyme system Red+Luc were reported by Kratasyuk et al. (1998a), and a method for the evaluation of wheat grain infection with Fusarium has been developed (Kratasyuk et al., 1998b). Also, a possibility was demonstrated to use bioluminescent bacterial systems for safety evaluations of food preservatives (Asanova et al., 2014). Nevertheless up till now, methods based on the use of the bioluminescent enzymatic bacterial systems have not been widely utilized for the evaluation of food product quality.
Bioluminescent enzymatic toxicity assays are also very promising for use in medical research, for example for evaluating the gravity of endotoxicosis during treatment in surgery and therapy. This is based on the fact that the effect of the blood serum of donors on this assay differs markedly from that of patients. It has been shown that the blood serum of a patient inhibits bioluminescence less than that of a donor.
Comparative analysis of the usefulness of the luciferase index (LI), and other laboratory parameters, to assess patients with peritonitis, have also been made (Sovtsov and Kratasyuk, 1991). The LI was useful in the case of doubt about the location of the abdominal inflammation, as well as judging the severity of the disease. Bioluminescent assays allow the estimation of a patient’s condition as satisfactory, of mildly serious, severe, or critical. The assays can be used also for predicting the course of the disease, estimation of the efficiencies of the used detoxification methods, and of the drainage procedure with semipermeable membranes. Most important is applying LI in a prognostic plan, since the long low-positive LI dynamics could indicate the need for a change of treatment plan (Figure 5).
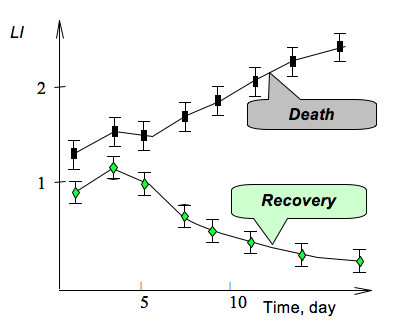
Figure 5. Effect of patients’ serum blood on the bioluminescence LI from the coupled enzyme system Red + Luc, indicating a safe recovery from the disease or an unfavorable course.
It was reported that bioluminescent enzymatic toxicity assay can be used as a reliable criterion to monitor the course of disease for patients undergoing therapy for bronchitis, peptic ulcer, and chronic cholecystitis (Esimbekova et al., 1999). The most important advantages of the proposed approach are the very short time interval between sample collection and results, high sensitivity, low traumatism, and simplicity of the method.
A very interesting and promising trend in the development of bioluminescent enzymatic toxicity assay is the design of rapid analysis for the assessment of human organism reaction to physical and mental stress. Analysis is made by comparing the light emission intensity of the coupled enzyme system Red + Luc in the presence of a person’s saliva taken before and after a physical stress. The main advantages of this method are not only its rapidity, high precision, and sensitivity, but also noninvasiveness, because only human saliva is analyzed, which reflects the functional state of a person just as blood does.
It is possible to use bioluminescent assay in vitro for selective analysis also, for example to measure stress in plants. Pyridine nucleotides are key redox carriers in the soluble phase of all living cells, and play crucial roles in pro- and antioxidant metabolism, and non-redox mechanisms, in particular, in plant stress responses. Therefore, the determination of pyridine nucleotide content and the ratio of their oxidized and reduced forms can be the basis for monitoring of plant response to stress caused by environmental changes. A bioluminescence method to determine pyridine nucleotide NAD(P)H, NAD(P)+ in plant tissues grown in closed hypobaric growth chambers have been developed. The experiments model conditions that would be used for plant chambers on the Moon or Mars as indicators of oxidative stress caused by a low pressure environment (Kratasyuk et al., 2011) (Figure 6).

Figure 6. (A) Growing of the plant samples in climate-controlled chambers; (B) Concentrations of NAD(P)+ and NAD(P)H in the extracts of radish roots under conditions of modeled stress: control – sample plants grown at 101.5 kPa; (1) experimental plants grown at 32 kPa and oxygen partial pressure 4.7 kPa for 24 h; (2) experimental plants grown at 32 kPa and oxygen partial pressure 6.5 kPa for 24 h and then additionally at the 9 kPa pressure and oxygen partial pressure 3.3 kPa for the next 24 h.
Enzymatic Reagents for Bioluminescent Analysis
Widespread use of the available bioluminescent enzymatic toxicity bioassays would be limited by the instability of the enzymes during use, limited shelf-life of enzymes and reagents, the need to control ambient conditions (i.e., pH, temperature, etc.), high manufacturing cost, and other factors. These problems are overcome by using immobilized enzymes that possess high catalytic activity and stability for long-term storage, and successfully serve as biological modules for biosensors (Turner, 2000).
For the last 30 years, immobilization has been widely used for the production of stable reagents for bioluminescent analysis, based on various bioluminescent systems: luminous bacteria, and bacterial and firefly luciferases. Many of the available immobilized reagents are successfully used in analytic measurements and in biosensors, because they simplify the analysis procedure, sometimes enabling full automation. At present, there are more than 40 different methods of immobilizing luminous organisms and enzymes (Kratasyuk and Esimbekova, 2003). An important advantage of immobilized enzymes is the possibility to control the enzyme stability against physical and chemical factors by choosing a suitable microenvironment. The optimal microenvironment for bacterial luciferase is natural polymer gels such as gelatin or starches (potato, rice, or corn). By varying gel concentration, time, and mode of drying of immobilized enzymes, it is possible to make reagents with different enzymatic activity (Esimbekova et al., 2007, 2015).
It was shown that the coupled enzyme system Red + Luc immobilized in starch or gelatin gel, preserves its activity for 2 years (Lonshakova-Mukina et al., 2015). Moreover, immobilization in these gels leads to a considerable stabilization of the coupled enzyme system with regard to denaturation treatment: pH optimum of the enzymes expands both to the acid and alkaline regimes; high enzyme activity is maintained at increased salt concentration; thermal stability also increases especially in the case of starch gel immobilization (Esimbekova et al., 2009; Bezrukikh et al., 2014).
Several substrates of the bacterial bioluminescent reaction can be co-immobilized together with the coupled enzyme system to make the final test much simpler. For example, a homogeneous multicomponent reagent named Enzymolum contains the enzymes Red and Luc, their substrates (myristic aldehyde and NADH), and buffer salts, co-immobilized in starch or gelatin gel (Kratasyuk and Esimbekova, 2011). The reagent is currently produced in flake form and can be contained within the bioluminometer cuvette (Figure 7).

Figure 7. The multicomponent reagent Enzymolum is a flake of dried gel, diameter 6–7 mm; dry weight 1.5 ± 0.2 mg.
The advantages of enzymatic assays using Enzymolum are rapidity (the time of analysis does not exceed 3–5 min), high sensitivity, a one-step measuring procedure, and the possibility of automation (Figure 8).
 Figure 8. Scheme of toxicity bioassay with the reagent Enzymolum.
Figure 8. Scheme of toxicity bioassay with the reagent Enzymolum.
Conclusion
We describe here a new approach in developing bacterial bioluminescent enzymatic biosensors, application to toxicity bioassays, and the needed reagents. To solve the problem of how to detect, identify, and measure the numerous chemical compounds in environmental monitoring, food product contamination, and medical diagnostics, these bioluminescent enzymatic toxicity assays are proposed, wherein the coupled enzyme system NAD(P)H:FMN-oxidoreductase and bacterial luciferase, substitutes for the older methods using living organisms. The immobilized reagent Enzymolum was introduced to facilitate and accelerate the development of cost-competitive enzymatic systems for use in biosensors for toxicological assays. The reagent is easy to use and convenient to be applied not only in toxicology studies but also in education, mainly in ecology and enzymology practical courses (Gitelson and Kratasyuk, 2002, Kratasyuk and Kudinova, 1999). Prototype biosensors offer cost advantages, versatility, high sensitivity, rapid response, extended shelf-life, and flexible storage conditions.
[NOTE: For detailed information on topics provided in this chapter, please refer to Esimbekova et al. (2014). A commercial source of Enzymolum is Prikladnye Biosistemy Ltd. (pr. Svobodnii, 79, room 13-08, Krasnoyarsk, 660036, Russia, e-mail: valkrat@mail.ru).
This work was supported by a state contract between the Ministry of Education and Science and the Siberian Federal University, №1762, 2014-2015.
References
Asanova, A., Esimbekova, E., Kratasyuk, V. (2014) Bioluminescent Enzymatic Methods for Toxicological Safety Testing of Food Additives. Luminescence 29:74.
Bezrukikh, A., Esimbekova, E., Nemtseva, E., Kratasyuk, V., Shimomura, O. (2014) Gelatin and starch as stabilizers for the coupled enzyme system of luminous bacteria NADH:FMN-oxidoreductase-luciferase. Anal Bioanal Chem 406:5743-5747.
Dubovskaya, O.P., Gladyshev, M.I., Esimbekova, E.N. et al. (2002) Study of possible relation between seasonal dynamics of zooplankton nonconsumptive mortality and water toxicity in a pond. Inland Water Biol 3:39–43.
Esimbekova, E.N., Kondik, A.M., Kratasyuk, V.A. (2013) Bioluminescent enzymatic rapid assay of water integral toxicity. Environ Monit Assess 185:5909–5916.
Esimbekova, E.N., Kratasyuk, V.A., Abakumova, V.V. (1999) Bioluminescent method nonspecific endotoxicosis in therapy. Luminescence 14:197–198.
Esimbekova, E., Kratasyuk, V., Shimomura, O. (2014) Application of enzyme bioluminescence in ecology. Adv Biochem Eng Biotechnol 144:67-109.
Esimbekova, E.N., Kratasyuk, V.A., Torgashina, I.G. (2007) Disk-shaped immobilized multicomponent reagent for bioluminescent analyses: correlation between activity and composition. Enzyme Microb Tech 40:343–346.
Esimbekova, E.N., Lonshakova-Mukina, V.I., Bezrukikh, A.E., Kratasyuk, V.A. (2015) Design of multicomponent reagents for enzymatic assays. Dokl Biochem Biophys 461.
Esimbekova, E.N., Torgashina, I.G., Kratasyuk, V.A. (2009) Comparative study of immobilized and soluble NADH:FMN-oxidoreductase-luciferase coupled enzyme system. Biochemistry (Moscow) 74:695–700.
Fernández-Piñas, F., Rodea-Palomares, I., Leganés, F. et al. (2014) Evaluation of the ecotoxicity of pollutants with bioluminescent microorganisms. Adv Biochem Eng Biotechnol 145:65-135.
Gitelson, J.I., Kratasyuk, V.A. (2002) Bioluminescence as an educational tool. In: Kricka LJ, Stanley PE (eds) Bioluminescence and chemiluminescence: progress and current applications. World Scientific Publishing Co., River Edge, pp. 175–182.
Girotti, S., Ferri, E.N., Fumo, M.G. et al. (2008) Monitoring of environmental pollutants by bioluminescent bacteria. Anal Chim Acta 608:2–29.
Girotti, S., Muratori, M., Fini, F. et al. (2000) Luminescent enzymatic flow sensor for D- and L-lactate assay in beer. Eur Food Res Technol 210:216–21.
Kratasyuk, V.A. Principle of luciferase biotesting. In: Biological luminescence, Proceedings of the first international school, Wroclaw, Poland, June 20-23, 1989; World Scientific Publishing Co., Singapore, 1990; pp. 550-558.
Kratasyuk, V.A., Gitelson, J.I. (1987) Application of luminous bacteria in bioluminescent analysis. Uspekhi microbiologii 21:3–30.
Kratasyuk, V.A., Egorova, O.I., Esimbekova, E.N. et al. (1998b) A biological luciferase test for the bioluminescent assay of wheat grain infection with Fusarium. Appl Biochem Micro (Moscow) 34:622–624.
Kratasyuk, V.A., Esimbekova, E.N. (2003) Polymer immobilized bioluminescent systems for biosensors and bioinvestigations. In: Arshady R (ed) Polymeric biomaterials, The PBM Series (Introduction to Polymeric Biomaterials), vol 1. Citus Books, London, pp 301–343.
Kratasyuk, V.A., Esimbekova, E.N. (2011) Bioluminescent biomodule for analyses of various media toxicity and method of its preparation. RU Patent 2,413,772, 10 Mar 2011.
Kratasyuk, V., Esimbekova, E., Correll, M., Bucklin, R. (2011) Bioluminescent enzyme assay for the indication of plant stress in enclosed life support systems. Luminescence 26:543-546.
Kratasyuk, V.A., Esimbekova, E.N., Gladyshev, M.I. et al. (2001) The use of bioluminescent biotests for study of natural and laboratory aquatic ecosystems. Chemosphere 42:909–915.
Kratasyuk V.A., Kudinova I.Y. (1999) Practical enzymology course based on bioluminescence. Luminescence 14:189-192.
Kratasyuk, V.A., L’vova, L.S., Egorova, O.I. et al. (1998a) Effect of Fusarium mycotoxins on bacterial bioluminescence system in vitro. Appl Biochem Micro (Moscow) 34:190–192.
Kratasyuk, V.A., Vetrova, E.V., Kudryasheva, N.S. (1999) Bioluminescent water quality monitoring of salt Lake Shira. Luminescence 14:193–195.
Kudryasheva, N.S. (2006) Bioluminescence and exogenous compounds. Physico-chemical basis for bioluminescent assay. J Photochem Photobiol, B 83:77–86.
Kudryasheva, N.S., Esimbekova, E.N., Remmel, N.N. et al. (2003) Effect of quinones and phenols on the triple—enzyme bioluminescent system with protease. Luminescence 18:224–228.
Kudryasheva, N.S., Kudinova, I.Y., Esimbekova, E.N. et al. (1999) The influence of quinones and phenols on the triple NAD(H)-dependent enzyme systems. Chemosphere 38:751–758.
Lin, L.Y-C., Meighen, E.A. (2009) Bacterial Bioluminescence: Biochemistry and Molecular Biology, on Photobiological Sciences Online (KC Smith, ed.). American Society for Photobiology, http://www.photobiology.info/.
Lonshakova-Mukina, V., Esimbekova, E., Kratasyuk, V. (2015) Impact of enzyme stabilizers on the characteristics of biomodules for bioluminescent biosensors. Sensor Actuat B-Chem 213:244–247.
Mei, C., Wang, J., Lin, H. et al. (2009) Quantitative detection of NADH by in vitro bacterial luciferase bioluminescent. Wei Sheng Wu Xue Bao 49:1223–1228.
Njus, D., Baldwin, T.O., Hastings, J.W. (1974) A sensitive assay for proteolytic enzymes using bacterial luciferase as a substrate. Anal Biochem 61:280–287.
Peng, Y., Jin, Y., Lin, H. et al. (2014) Application of the VPp1 bacteriophage combined with a coupled enzyme system in the rapid detection of Vibrio parahaemolyticus. J Microbiol Meth 98:99–104.
Petushkov, V., Shefer, L., Rodionova, N. et al. (1987) Bioluminescent method of determination of NAD(P)H dehydrogenase activity. Appl Biochem Biotech 23:270–274.
Rimatskaia, N., Baigina, E., Kazanceva, M., et al. (2014) Application of bioluminescent enzymatic method for assessment of the state of the soil. Luminescence 29:66-67.
Rimatskaya, N.V., Nemtseva, E.V., Kratasyuk, V.A. (2012) Bioluminescent assays for monitoring of air pollution. Luminescence 27:154.
Roda, A., Guardigli, M., Michelini, E. et al. (2009) Bioluminescence in analytical chemistry and in vivo imaging. Trends Anal Chem 28:307–322.
Shimomura, O. (2006) Bioluminescence: chemical principles and methods. World Scientific Publishing Co., Singapore.
Shishatskaya, E.I., Esimbekova, E.N., Volova, T.G. et al. (2002) Hygienic assessment of polyhydroxyalkanoates—natural polyethers of new generation. Gigiena Sanitaria 4:59–63.
Sovtsov, S.A., Kratasyuk, V.A. (1991) Determination of endotoxicosis under surgery. USSR Patent 1,714,512, 22 Oct 1991.
Tarasova A.S., Stom D.I., Kudryasheva N.S. (2015) Antioxidant activity of humic substances via bioluminescent monitoring in vitro. Environ Monit Assess 187:89.
Turner, A.P.F. (2000) Biosensors—sense and sensitivity. Science 290:1315–1317.
Xu, T., Close, D., Smartt, A. et al. (2014) Detection of organic compounds with whole-cell bioluminescent bioassays. Adv Biochem Eng Biotechnol 144:111-151.
Vetrova, E., Esimbekova, E., Remmel, N. et al. (2007) A bioluminescent signal system: detection of chemical toxicants in water. Luminescence 22:206–214.
Vetrova, E.V., Kratasyuk, V.A., Kudryasheva, N.S. (2002) Bioluminescent characteristics of Shira Lake water. Aquat Ecol 36:309–315.
09/18/15