INTRODUCTION TO PHOTODYNAMIC THERAPY
David Kessel
Department of Pharmacology
Wayne State University School of Medicine
Detroit, MI 48201
dhkessel@med.wayne.edu
www.med.wayne.edu/pharm/pharm3/Kessel.asp
Background
In 1900, a German medical student in Munich named Oskar Raab discovered that when paramecia were examined under a microscope, in the presence of different dyes, the cells gradually lost motion and were unable to divide. This was termed by his mentor, Hermann von Tappiener, a "Photodynamische" or "photodynamic effect", indicating that light played a critical role. In later publications, von Tappiener suggested that this effect might be used in clinical medicine.
[See Historical Vignette]
Photodynamic therapy (PDT) was periodically reinvented at intervals thereafter. One notable rediscovery was reported at the first ASP meeting (1973) by McDonaugh, Nielsen, Wolson and Jaenicke. When hematoporphyrin was added to glioma cells in culture, cell death was observed upon irradiation of the cultures with light from a bank of fluorescent bulbs. They also reported that a tumor produced by subcutaneous injection of glioma cells into rats could be eradicated by light from a 150-watt bulb. This was duly reported in the Sarasota (Florida) Journal of 14 June 1973, under the headline: "Simple light bulb killed cancer cells in experiments".
In the early 1970s, the clinical application of the photodynamic effect was progressing at the Roswell Park Cancer Center under the direction of Thomas Dougherty and associates, using a preparation described earlier by Dr. Sam Schwartz, and termed HPD (hematoporphyrin derivative). Schwartz was initially searching for a radiosensitizer, as have numerous others in the ensuing years. During the course of this work, he had observed that crude preparations of hematoporphyrin tended to localize at sites of neoplasia, where their fluorescence could be detected by UV light. Schwartz empirically attempted to produce a better porphyrin, and eventually hit on the idea of treating hematoporphyrin with a mixture of acetic + sulfuric acids, pouring the resulting red-brown solution into water, filtering off the product which was then dissolved in dilute NaOH. Since the structure of this material was unknown, Schwartz called it HPD (hematoporphyrin derivative).
Dougherty was also seeking a radiation sensitizer, and consulted with Schwartz concerning the possibilities that HPD might be a useful agent. Dougherty soon discovered that HPD was far more useful as a photosensitizing agent. It had already been established that the photodynamic effect needed light at a wavelength corresponding to an absorbance band of the sensitizer, but also one that could penetrate through tissues. The soret band (~400 nm) can penetrate for about one cell-depth, hardly adequate for therapeutic purposes, so it was necessary to use the much weaker porphyrin absorbance band at 630 nm. In the early studies, patients were given an intravenous dose of a sterile HPD solution and then exposed to a bright red light. Dougherty and his group achieved considerable success, and the news gradually spread to other sites. In 1977, a "users" meeting was organized at Roswell Park in Buffalo, the NIH sponsored another such meeting 2 years later, with many more to come.
PDT: mechanisms and clinical successes
PDT, as presently practiced turns out to be useful for the treatment of many types of neoplastic disease. The major limitations are getting sufficient light, oxygen and drug to the site. In order for the pertinent protocols to be developed, considerable pre-clinical research and toxicity studies are needed before initiating clinical studies. While PDT can kill malignant cells, an important effect is also the shut-down of the tumor vasculature. This can provide a means for killing several logs of cells, while direct tumor cell kill alone may kill only 1-3 logs. The photodynamic effect requires light. Insufficient light will yield a poor result. It is therefore necessary to insure that all of the tumor is being irradiated. While surface illumination may be adequate for many indications, e.g., skin lesions, the usual approach involves a laser-fiber optic combination, with multiple fibers often inserted into lesions.
The efficacy of PDT derives from a light-induced conversion of molecular oxygen via energy transfer from the excited state of the photosensitizer into a highly-reactive species termed "singlet oxygen". This material will interact with the first biological molecule it sees, and will therefore not wander through the circulation causing damage at remote sites. This phenomenon is responsible for the highly-localized toxic effect of PDT. It is necessary for the tissues to be well-oxygenated for PDT to be successful. Hypoxic tissues will be protected, and the vascular shut-down that accompanies PDT can also be a limiting factor. The fluorescence of the tumor-localizing photosensitizing agents can be of substantial assistance in locating tumor sites. This has been accomplished in the lung, bladder, oral cavity and other locations via endoscopy, and in the examination of skin lesions under UV light.
The explanation for the localization of photosensitizers at tumor loci is still unclear. The effective agents tend to bind to lipoproteins, and receptors for lipoproteins do tend to be up-regulated in neoplastic cells. Photosensitizers do accumulate in some normal tissues with high levels of lipoprotein receptors, e.g., the adrenal cortex, the pituitary and embryonic cells, but these are protected from photodamage, since they are not irradiated. Some organs, e.g., liver and spleen tend to accumulate photosensitizers, but are shielded from light during therapeutic procedures.
The nature of HPD and Photofrin
Considerable attention was given to the identification of the components of HPD, since this was the first photosensitizing agent to be examined, the first to be approved by regulatory agencies, and still remains in widespread use. The starting material is hematoporphyrin (HP, Figure 1) which is contaminated with variable amounts of dehydration products: hydroxyvinyl deuteroporphyrin (HVD) and protoporphyrin (PP). Note that there can be two positional isomers of HVD. Basic hydrolysis of the acetylated products yields a complex mixture containing the starting materials: HP, HVD and PP, along with a collection of dimers and higher oligomers joined by ether and ester linkages (Figure 1). Mass spec data indicates that oligimers containing 2-7 porphyrin units are present.
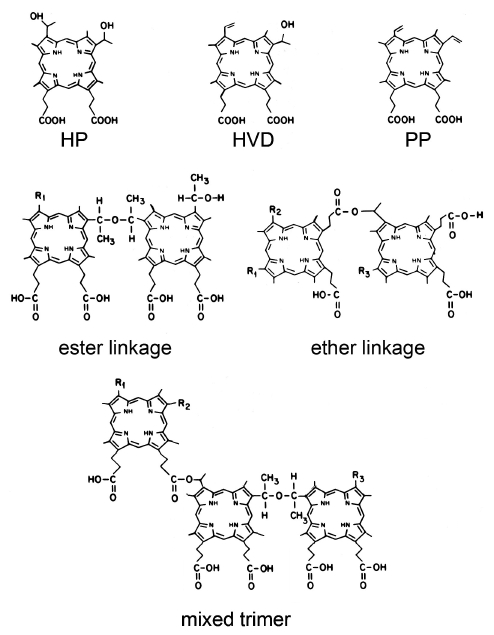
Figure 1. The PDT photosensitizer hematoporphyrin and its derivatives. Hematoporphyrin (HP), hydroxyvinyl deuteroporphyrin (HVD), and protoporphyrin (PP), and the porphyrin species found in HPD joined by ester or ether linkages, or both (mixed trimer).
The product currently being marketed under the name Photofrin® consists of HPD in which the percentage of monomers is greatly reduced. Since the ester linkages can be unstable, the mixture is maintained in a heated solution until hydrolysis is complete and the low molecular-weight depleted. The remained is lyophilized for clinical use. The predominant species present appears to be a trimer with the porphyrin units joined by ether linkages. Unlike some of the newer photosensitizing agents, Photofrin® (like HPD) does cause a persistent photosensitization of the skin. This required avoidance of bright sunlight for at least 30 days after drug administration. Later sensitizers had many more favorable properties, including much more substantial absorbance at longer wavelengths, along with a very limited period of skin photosensitization. These were all single compounds, not complex mixtures. However, it should be noted that to date Photofrin® is one of the few photosensitizers approved for the treatment of cancer (early and advanced lung and esophageal cancers) by the US FDA, as well as several other health agencies worldwide. Foscan has been approved in Europe for head and neck cancer, and aminolevulinic acid (ALA), a biologic precursor of protoporphyrin IX, has been approved for some dermatologic uses.
How it works
The most effective photosensitizing agents localize in the endoplasmic reticulum, mitochondria or lysosomes of mammalian cells, sometimes combinations of these loci. Irradiation then brings about a series of steps leading to cell death, depending on where the damage occurs.
Photodamage to the endoplasmic reticulum or mitochondria results in loss of function of anti-apoptotic members of the Bcl-2 family. These proteins keep under control a collection of pro-apoptotic proteins, and loss of function generally leads to the release of pro-apoptotic proteins, which tend to have short names beginning with "B". Examples are Bad, Bax, Bik and Bim. These small proteins then bind to the mitochondrial membrane, creating pores that are permeable to the mitochondrial protein cytochrome c. The release of cytochrome c into the cytosol is one signal for the cell to initiate a death pathway termed "apoptosis".
Apoptosis is a strongly conserved route to death, and results in activation of endonucleases, cleavage of DNA into fragments, and activation of other proteases (caspases) that lead to cell fragmentation into particles that are then ingested by adjoining cells. The result is a non-inflammatory process that disposes of the dead cells. In contrast, necrosis results in the loss of the cell membrane and the release of lysosomal proteases and DNA into the environment, resulting in an inflammatory response. IL-6 production and inflammation occur at all effective treatment regimens, and these phenomena are believed to be responsible for the immunological effects and adaptive response that play an important role in tumor destruction.
Photodamage to lysosomes has the same end result, but via a different route. The release of lysosomal proteases into the cytosol results in the cleavage of the small-molecules called Bid. Bid is thereby converted to "truncated-Bid" (tBid). This protein can also open pores in the mitochondrial membrane, leading to apoptosis.
Photosensitizers that bind to the cell membrane tend to be weakly effective in animal studies. During irradiation, these agents tend to migrate into the cytosol as the membrane is altered. Once in the cytosol, they cause photo-inactivation of some of the caspases involved in the apoptotic process. As a result, apoptosis is impaired, and the cells can only die via necrosis. This is a much less efficient process than apoptosis, since caspase activation is autocatalytic. In terms of photons absorbed per cells killed, any process that relies on necrosis as a death pathway is perhaps 10% as efficient as a process involving apoptosis.
Some recent studies have indicated that autophagy is also involved in PDT-induced cell death. Autophagy is a process whereby cells starved for nutrients can recycle their components and delay cell death. This is done by a process that involves formation of vacuoles that encircle the components to be recycled. These fuse with lysosomes, use the lysosomal proteases to degrade the contents of the vacuole to amino acids and other starting materials, and then release these back into the cytosol.
After photodamage, cells can also recycle photodamaged organelles via autophagy. This appears to account for the "shoulder" on the dose-response curve. If autophagy is inhibited, a low dose of light becomes much more effective than where autophagy is present. Autophagy can also play a role in cell death after PDT. This occurs when cells attempt to carry out "too much" autophagy. They become filled with autophagic vacuoles, but there are not enough functional components of the cell to utilize the products.
A second factor is the loss of the tumor vasculature after PDT. This deprives the cells of oxygen and other nutrients, and therefore enhances the net phototoxic effect of PDT. One of unexpected features of PDT is the use of its capacity for vascular shutdown to treat macular degeneration. This disease involves the loss of central vision as the retina of the eye becomes overgrown with small blood vessels. PDT can eradicate these vessels and prevent further loss of vision.
Why is PDT useful? How can it be improved?
It is common for tumor cells to remain at surgical margins. PDT can eradicate such cells, promoting the long-term survival of cancer patients. There are also indications that PDT can help control local bacterial and fungal infections. Current research is directed at specific problems in drug and light delivery.
Newer sensitizers that do not cause persistent skin photosensitization, and have strong absorbance bands at wavelengths >700 nm, are being identified. Among these are mTHPC (meta tetrahydroxyphenyl chlorin, absorbance at 650 nm) and BPD (benzoporphyrin derivative, absorbance at 690 nm), shown in Figure 2. Both of these structures are based on a central porphyrin ring system, as is indicated by the dark outline.
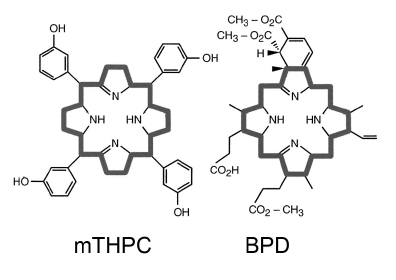
Figure 2. PDT photosensitizers: meta tetrahydroxyphenyl chlorin (mTHPC), and benzoporphyrin derivative (BPD). Both of these structures are based on a central porphyrin ring system, as is indicated by the dark outline.
Transient skin photosensitization is not really a serious problem, but can be a nuisance since patients are required to avoid strong sunlight for sometimes as long as 60 days.
Better long-wavelength absorbance promotes light delivery to deep tumors. Procedures are being developed for improving light delivery to larger tumor sites, e.g., the prostate. Multiple fibers need to be inserted with feedback mechanisms in place to monitor the uniformity of light delivery. While macular degeneration is currently being treated with an antibody to VEGF, the protein that elicits abnormal vessel growth, there is evidence that a combination of anti-VEGF therapy + PDT will yield a better result.
New uses for PDT are constantly being sought. There are groups investigating the potential for treatment of arthritis, atherosclerosis and other problems, where delivery of therapy to a specific location may be of help. Among the synthetic projects is the development of "bifunctional" agents that have both a highly-fluorescent component (for tumor detection) and a moiety that is very efficient at producing singlet oxygen upon irradiation (for tumor eradication). This permits both easy localization of neoplasia and its treatment. Another area under investigation is the combination of PDT with other procedures. Biel has been successful in using a combination of surgery + PDT for the treatment of head & neck tumors. Surgery often leaves tumor cells at the margins, and the adjuvant use of PDT can often eradicate such cells. Hasan's group is examining the ability of PDT to act synergistically with chemotherapy in cancer control. Studies on the use of nanotechnology to enhance the efficacy and selectivity of PDT are also being examined by this group, as well as others.
Further information on PDT can be found in the following references. Note the descriptions in the brackets. Additional information can also be found in the module on Basic Photomedicine.
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 61,250-281. 2011. [This is the most recent comprehensive review of clinical implications for PDT.]
Biel M. Advances in photodynamic therapy for the treatment of head and neck cancers. Lasers Surg Med. 38:349-55, 2006. [A report on the use of PDT for removal of tumors at surgical margins.]
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy.J Natl Cancer Inst. 90:889-905, 1998. [This review contains a summary of PDT progress in a variety of fields.]
Henderson BW, Dougherty TJ. Photodynamic Therapy. Marcel Dekker: New York, 1992. [This is a good introductory textbook and contains a section written by Tom Dougherty, Sam Schwartz, Robert Lipson and James Winkelman, describing the early history of PDT.]
Jori G, Reddi E. The role of lipoproteins in the delivery of tumour-targeting photosensitizers. Int J Biochem. 25:1369-75, 1993. [One of the first reports linking porphyrin localizatino in neoplastic tissues with a lipoprotein-directed process.]
Kabingu E, Vaughan L, Owczarczak B, Ramsey KD, Gollnick SO. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br J Cancer. 96:1839-48, 2007. [A discussion of the role played by immunologic phenomena as a factor in PDT efficacy.]
Kessel D. Selected papers on Photodynamic Therapy. SPIE Milestone Series Vol MS 82. BJ Thompson, Ed., SPIE 1993. [This contains a series of original papers on PDT between 1948 and 1992.]
Kessel D, Reiners JJ Jr. Apoptosis and autophagy after mitochondrial or endoplasmic reticulum photodamage. Photochem Photobiol. 83:1024-8, 2007. [A description of the assorted death pathways evoked by PDT.]
MacCormack MA. Photodynamic therapy in dermatology: an update on applications and outcomes. Semin. Cutan. Med. Surg. 27:52-62, 2008. [A summary of PDT applications in Dermatology.]
Mir Y, Elrington SA, Hasan T. A new nanoconstruct for epidermal growth factor receptor-targeted photo-immunotherapy of ovarian cancer. Nanomedicine. 9, 1114-1122, 2013. [An exanple of the use of nanotechnology for promoting PDT selectivity].
Overholt BF, Wang KK, Burdick JS, Lightdale CJ, Kimmey M, Nava HR, Sivak MV Jr, Nishioka N, Barr H, Marcon N, Pedrosa M, Bronner MP, Grace M, Depot M. International Photodynamic Group for High-Grade Dysplasia in Barrett's Esophagus. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc. 66:460-8, 2007. [A comprehensive report on the use of PDT for therapy of esophageal dysplasia.]
Pandey RK, Siegel MM, Tsao R, McReynolds JH, Dougherty TJ. Fast atom bombardment mass spectral analyses of Photofrin II and its synthetic analogs.Biomed Environ Mass Spectrom. 19:405-14, 1990. [The first detailed analysis of the porphyrins that are found in HPD. See Figure 1.]
Reiners JJ Jr, Agostinis P, Berg K, Oleinick NL, Kessel D. Assessing autophagy in the context of photodynamic therapy. Autophagy. 6, 7-18, 2010. [This provides a review of the role of autophagy as a survival or death mechanism after cellular photodamage].
Verma S, Watt GM, Mai Z, Hasan T. Strategies for enhanced photodynamic therapy effects. Photochem Photobiol. 83:996-1005, 2007. [Potential for use of PDT in combination with other modalities.]
08/14/08
03/01/10
03/29/14