BACTERIAL PHOTOSYNTHESIS
Mike Jones
Department of Biochemistry, School of Medical Sciences
University of Bristol, University Walk, Bristol, BS8 1TD, United Kingdom
m.r.jones@bristol.ac.uk
1. Introduction
Although the process of photosynthesis is most commonly associated with plants and algae, much of our understanding of the molecular basis for light energy capture and photochemical energy transduction has come from studies of photosynthetic bacteria. Space precludes a detailed description of the different types of photosynthetic bacteria, and so this article will focus in the main on the most heavily studied group of anoxygenic phototrophs, with only brief mention of their less well-known cousins. The cyanobacteria, which carry out a process of oxygenic photosynthesis common to that found in green plants and algae, are not covered in this article.
Even with a limited focus the subject of bacterial photosynthesis is vast, and so the following description is necessarily selective, focusing on the most heavily-studied systems and key issues, and inevitably reflecting the author's personal bias. Accordingly, the bibliography at the end of the article includes some general works that provide more detailed and comprehensive information, and in some instances, in-the-text citations are to recent review articles rather than original research papers. Regarding general works, in particular, the reader is guided to a book by Blankenship (2002), which provides a detailed and accessible account of the light reactions of photosynthesis, and the underlying physical chemistry, and also to a recent book edited by Hunter et al. titled "The Purple Photosynthetic Bacteria" (2008), which contains authoritative reviews on many of the aspects of bacterial photosynthesis outlined below.
2. Energy Transduction in the (Anoxygenic) Photosynthetic Bacteria
The bacteria that use chlorophyll-type molecules to exploit sunlight as an energy source comprise the purple phototrophic bacteria, green sulphur bacteria, green filamentous bacteria, the heliobacteria and the cyanobacteria. All but the last of these transduce light energy into a biologically-useful form without the generation of oxygen from the oxidation of water, and are classed as anoxygenic photosynthetic bacteria. The most heavily-studied group are the purple phototrophic bacteria, for a variety of reasons including metabolic flexibility, genetic accessibility, and the relatively simple and modular nature of their photosynthetic apparatus.
In general terms, the strategy for solar energy utilization in all organisms that contain chlorophyll (Chl) or bacteriochlorophyll (BChl) is the same.
1. Light energy is captured by pigment molecules in the light harvesting or "antenna" region of the photosystem, and is stored temporarily as an excited electronic state of the pigment.
2. Excited state energy is channeled to the reaction centre region of the photosystem, a pigment-protein complex embedded in a charge-impermeable lipid bilayer membrane.
3. Arrival of the excited state energy at a particular bacteriochorophyll (BChl), or pair of BChls in the reaction centre triggers a photochemical reaction that separates a positive and negative charge across the width of the membrane.
4. Charge separation initiates a series of electron transfer reactions that are coupled to the translocation of protons across the membrane, generating an electrochemical proton gradient [protonmotive force (pmf)] that can be used to power reactions such as the synthesis of ATP.
The minimal photosynthetic unit in purple phototrophic bacteria comprises a reaction centre (RC) surrounded by a light harvesting complex called LH1. Together these form the so-called RC-LH1 complex, and this is capable of converting light energy into a pmf in partnership with a second membrane-embedded electron transfer protein, the cytochrome (cyt) bc1 complex (termed bc1 below). In some species, light harvesting capacity is augmented by one or more types of peripheral antenna complex, termed LH2, LH3, and so on. A number of general reviews on the structure and mechanism of the purple bacterial photosystem have been published (Hu et al., 1998; Sundström et al., 1999; Hu et al,, 2002; Cogdell et al., 2004; Law et al., 2004; Cogdell et al., 2006; Sundström, 2008).
3. Component Structure
Pigments. The main light harvesting pigment in purple photosynthetic bacteria is not chlorophyll but bacteriochlorophyll (BChl), a closely related magnesium porphyrin that has a more saturated tetrapyrrole ring (Figure 1A). This causes BChl to absorb at significantly longer wavelengths than chlorophyll in the near infrared, the absorbance spectrum being dictated by the details of the conjugated
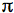 electron system of the macrocycle (Blankenship, 2002) (Figure 1A). Light harvesting is also carried out by a variety of carotenoids that provide the main pigmentation in the visible region of the spectrum, and so make purple bacteria purple (or a variety of other colours). Figure 1B shows the absorbance spectrum of photosynthetic membranes prepared from Rhodobacter (Rba.) sphaeroides; the strong absorbance in the near infrared is attributable to BChl (Qy bands) as is the band around 600 nm (Qx bands), whilst absorbance between 450 and 550 nm arises mainly from carotenoid. The so-called Soret bands of BChl are located in the near-UV between 300 and 400 nm (not shown).
electron system of the macrocycle (Blankenship, 2002) (Figure 1A). Light harvesting is also carried out by a variety of carotenoids that provide the main pigmentation in the visible region of the spectrum, and so make purple bacteria purple (or a variety of other colours). Figure 1B shows the absorbance spectrum of photosynthetic membranes prepared from Rhodobacter (Rba.) sphaeroides; the strong absorbance in the near infrared is attributable to BChl (Qy bands) as is the band around 600 nm (Qx bands), whilst absorbance between 450 and 550 nm arises mainly from carotenoid. The so-called Soret bands of BChl are located in the near-UV between 300 and 400 nm (not shown).
Figure 1. Pigments and absorption.
(A) The structure of bacteriochlorophyll a. Carbon and oxygen atoms that form part of theelectron system are shown in yellow and red, respectively, with other carbon and oxygen atoms in white and orange. The central Mg (magenta sphere) is coordinated in-plane by the four pyrrole nitrogens (blue), plus a fifth out-of-plane ligand donated by the protein (not shown). The five rings are numbered as shown, and the labelled carbonyl groups provide additional potential points of attachment to the protein.
(B) Visible/near-infrared absorbance spectrum of photosynthetic membranes from Rba. sphaeroides.
Peripheral Antenna. X-ray crystallography and other structural techniques have revealed that the light harvesting pigment-proteins of purple bacteria have a cylindrical architecture. In Rhodopseudomonas (Rps.) acidophila the peripheral LH2 complex is formed from nine copies of each of two short polypeptides, termed
 and ß, that each have a single membrane-spanning
and ß, that each have a single membrane-spanning  -helix (Figure 2). These
-helix (Figure 2). These 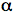 and ß polypeptides form two concentric protein cylinders in the membrane, between which are sandwiched the light harvesting BChls and carotenoids (McDermott et al. 1995).
and ß polypeptides form two concentric protein cylinders in the membrane, between which are sandwiched the light harvesting BChls and carotenoids (McDermott et al. 1995).
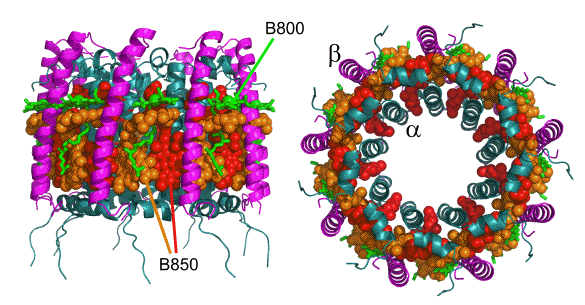
Figure 2. The LH2 pigment-protein from Rhodopseudomonas acidophila. Views are parallel (left) and perpendicular (right) to the plane of the membrane. The protein scaffold comprises concentric cylinders of nine(inner), and nine ß (outer) polypeptides (teal and purple ribbons, respectively). The 18 B850 BChls (which absorb strongly at 850 nm) are shown as spheres, in alternating red and orange, and the nine B800 BChls (which absorb strongly at 800 nm) are shown as green sticks (with the central Mg shown as a sphere). The macrocycles of the B850 and B800 BChls are arranged perpendicular and parallel, respectively, to the plane of the membrane. The figure was constructed using using Protein Data Bank (PDB) file 1NKZ (Papiz et al., 2003).
The BChls of LH2 form two rings that are arranged approximately in the plane of the membrane. The first of these comprises 18 BChls, one for each
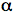 and ß polypeptide, oriented with the macrocyle of each BChl approximately perpendicular to the plane of the membrane (Figure 2). These "B850" BChls have a prominent absorbance band at 850 nm. The second ring comprises nine "B800" BChls, which are arranged with the macrocycle of each BChl parallel to the plane of the membrane, and gives rise to a prominent absorbance band at 800 nm. The LH2 complex also contains light harvesting carotenoids, although for clarity these are not shown in Figure 2. Peripheral LH complexes from other species show variations on this theme, e.g., the LH2 from Rhodospirillum molischianum has eight pairs of
and ß polypeptide, oriented with the macrocyle of each BChl approximately perpendicular to the plane of the membrane (Figure 2). These "B850" BChls have a prominent absorbance band at 850 nm. The second ring comprises nine "B800" BChls, which are arranged with the macrocycle of each BChl parallel to the plane of the membrane, and gives rise to a prominent absorbance band at 800 nm. The LH2 complex also contains light harvesting carotenoids, although for clarity these are not shown in Figure 2. Peripheral LH complexes from other species show variations on this theme, e.g., the LH2 from Rhodospirillum molischianum has eight pairs of 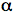 and ß polypeptides (Koepke et al., 1996). The LH2 from Rba. sphaeroides is very similar to that from Rps. acidophila, and its 850 and 800 nm absorbance bands are apparent in the spectrum shown in Figure 1B.
and ß polypeptides (Koepke et al., 1996). The LH2 from Rba. sphaeroides is very similar to that from Rps. acidophila, and its 850 and 800 nm absorbance bands are apparent in the spectrum shown in Figure 1B.
Core Antenna. To date a high resolution X-ray crystal structure for a RC-LH1 complex has not been reported, and the most detailed information available is from a 4.8 Å structure for the RC-LH1 complex from Rps. palustris (Roszak et al., 2003) (Figure 3). This shows a central RC surrounded by a cylindrical LH1 with a roughly oval cross section in the membrane plane. The LH1 pigment-protein has a similar overall architecture to LH2, being formed from concentric cylinders of two polypeptides also called
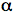 and ß. Each polypeptide has a single membrane-spanning
and ß. Each polypeptide has a single membrane-spanning 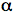 -helix, with a ring of BChls (one per
-helix, with a ring of BChls (one per 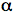 or ß polypeptide) sandwiched between the protein cylinders. LH1 is larger than LH2 to accommodate the RC in the central vestibule, with 15 pairs of
or ß polypeptide) sandwiched between the protein cylinders. LH1 is larger than LH2 to accommodate the RC in the central vestibule, with 15 pairs of 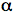 and ß polypeptides in Rps. palustris. LH1 contains carotenoids and single type of "B875" BChl, arranged in a ring with each BChl macrocycle perpendicular to the membrane. The 875 nm absorbance band of the Rba. sphaeroides LH1 complex can be seen as a shoulder in the spectrum in Figure 1B.
and ß polypeptides in Rps. palustris. LH1 contains carotenoids and single type of "B875" BChl, arranged in a ring with each BChl macrocycle perpendicular to the membrane. The 875 nm absorbance band of the Rba. sphaeroides LH1 complex can be seen as a shoulder in the spectrum in Figure 1B.
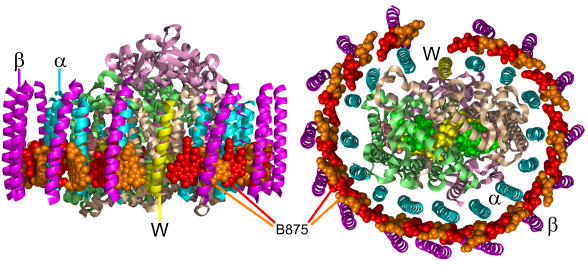
Figure 3. The RC-LH1 pigment-protein from Rps. palustris. Views are parallel (left) and perpendicular (right) to the plane of the membrane. The protein scaffold comprises concentric cylinders of 15(inner) and 15 ß (outer) polypeptides (teal and purple ribbons, respectively), plus a single W polypeptide (yellow ribbon). The 30 B875 BChls are shown as spheres, in alternating red and orange, their macrocycles being arranged perpendicular to the plane of the membrane. The central RC is shown as ribbons and spheres for polypeptides and cofactors, respectively (see Figure 4). The figure was constructed using PDB file 1PYH (Roszak et al., 2003).
In Rps. palustris, the ring of LH1 pigment-protein does not completely surround the central RC, and an additional membrane-spanning polypeptide is present in the gap in the LH1 ring. The function of this component is unclear, but it has been speculated that it is related to the PufX polypeptide, which is a minor component of the RC-LH1 complex in Rhodobacter (Rba.) sphaeroides, and is important for efficient quinone exchange between the RC and the bc1 (Holden-Dye et al., 2008).
Reaction Centre. The function of the carotenoids and BChls of the antenna is to feed the RC with excited state energy. The related PSO module by Yocum on RCs gives a detailed account of the structure, and mechanism of the purple bacterial RC. The following is a brief summary of the heavily-studied Rba. sphaeroides complex, included here for completeness. The module by Yocum describes the closely-related RC from Blastochloris (Blc.) viridis (Deisenhofer et al., 1995)
The Rba. sphaeroides RC comprises three polypeptides and ten cofactors, four BChls, two bacteriopheophytins (BPhe), which is BChl lacking the central Mg, two ubiquinones, a carotenoid and a non-heme iron (Allen et al., 1987) (Figure 4A). The BChl, BPhe and quinone cofactors are arranged within a protein scaffold formed by the L- and M-polypeptides in two approximately symmetrical membrane-spanning branches (Figure 4B). The arrival of excitation energy at a "special pair" of 870 nm-absorbing BChls (denoted P870) at the periplasmic end of the RC triggers a membrane-spanning four-step electron transfer along the A-branch of cofactors, which results in the reduction of a quinone at the so-called QB site near the cytoplasmic side of the membrane (Jones, 2009).
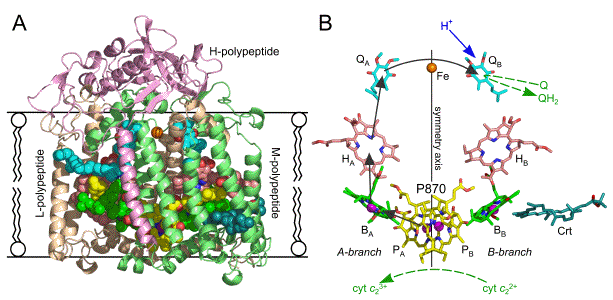
Figure 4. Overall structure and cofactors of the RC from Rba. sphaeroides.
(A) The cofactors (spheres) are encased by the largely intra-membrane L and M polypeptides (beige and light-green ribbons, respectively). The H polypeptide has an extra-membrane domain, and a single anchoring-helix (salmon ribbons).
(B) The cofactors are shown as sticks, with Mg and Fe atoms shown as purple or brown spheres, respectively. Hydrocarbon side chains of the BChl, BPhe and quinone cofactors are truncated for clarity. Carbon atoms are coloured thus: P870 BChl dimer (PA and PB) are yellow, monomeric BChls (BA and BB) are green, BPhes (HA and HB) are pink, quinones (QA and QB) are cyan, and carotenoid (Crt) is teal. Oxygens and nitrogens are shown in red and blue, respectively. The BChl, BPhe, and quinone cofactors are arranged in two membrane-spanning branches around an axis of two-fold pseudo-symmetry that connects P870 with the Fe atom. Black arrows show the route of electron transfer, blue arrow shows the site of H+ uptake, and dashed green arrows show Q/QH2 association/disassociation and delivery of electrons to P870+ by cyt c22+. The figure was constructed using PDB file 2BOZ (Potter et al., 2005).
As outlined below, the RC operates as a light-powered cytochrome c2:ubiquinone oxidoreductase, and participates in a cyclic protonmotive electron transfer system with a partner ubiquinol:cytochrome c2 oxidoreductase, the bc1 (Figure 5). This protein, which has similarities to the bc1 of mitochondria and the b6f complex of oxygenic phototrophs, catalyses a bifurcated electron transfer in which one electron removed from ubiquinol is used to reduce cyt c23+, and the second one is used to reduce quinone on the opposite side of the membrane (Hunte et al., 2003; Crofts, 2004). The X-ray crystal structure of the Rba. sphaeroides bc1 has been determined (Esser et al., 2008) and, in common with other bc1 complexes, this protein is dimeric (Figure 5A). At the core of the bc1 is a cyt b, comprising a membrane-spanning helix bundle encasing two hemes termed bL and bH. These form a membrane-spanning electron transfer chain that links a ubiquinol oxidase site near the periplasmic side of the membrane (Qo) with a quinone reductase site near the periplasmic side (Qi). The bc1 also contains a Rieske iron sulphur (Fe-S) protein containing a 2Fe-2S centre and a cyt c1, both of which have domains on the periplasmic side of the membrane, and a single membrane-spanning
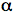 -helix. Each Rieske protein connects across the dimer, the membrane-spanning
-helix. Each Rieske protein connects across the dimer, the membrane-spanning 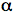 -helix associating with one monomer, and the extra-membrane domain associating with the second monomer.
-helix associating with one monomer, and the extra-membrane domain associating with the second monomer.
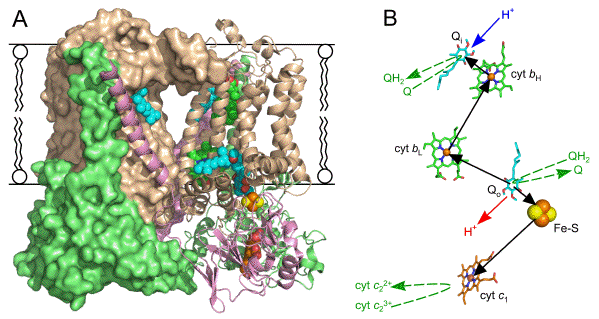
Figure 5. Overall structure and cofactors of the bc1 dimer from Rba. sphaeroides.
(A) In the view presented, parallel to the plane of the membrane, one half is shown as a solid object, and the second half as ribbons and spheres for the protein and cofactors, respectively. Each monomer comprises a cyt b (beige), cyt c1 (green) and Rieske Fe-S protein (pink).
(B) View in the plane of the membrane of a stick model of the cofactors of one half of the bc1 dimer. Heme Fe atoms are shown as small spheres, and the FeS centre as large spheres. Carbon atoms are coloured thus: b hemes are green, c heme is orange, and quinones are cyan. Oxygens, nitrogens, irons and sulphurs are shown in red, blue, brown and yellow, respectively. Black arrows show the routes of electron transfer, blue/red arrows show sites of proton uptake/release and dashed green arrows show association and disassociation of Q/QH2, and the reduction of cyt c23+ by cyt c1. The Figure was constructed using PDB file 2QJY (Esser et al., 2008), which has the inhibitor stigmatellin at the Qo site; this has been digitally replaced by quinone to construct the Figure.
4. Photosystem Mechanism
The purple bacterial photosystem is capable of harvesting light energy over a range of wavelengths (see above). Photon absorption produces a singlet excited electronic state of the absorbing pigment, and energy harvested by a carotenoid pigment of an antenna complex is transferred to an adjacent BChl (Sundström et al., 1999; Sundström, 2008). As outlined above, the BChls have three principal absorbance bands, the Soret band in the blue/near-UV region, the Qx band in the red region, and the Qy band in the near-infrared (the latter two are shown in Figure 1B). Energy absorbed in the Soret or Qx regions is converted to the lowest-energy Qy excited state through internal conversion.
The Qy excited state energies of the LH2 and LH1 BChls are arranged to ensure the funneling of energy into the RC. Within an individual LH2 protein, energy absorbed by the B800 BChls is passed to the ring of lower energy B850 BChls (Sundström et al., 1999). This energy is then passed either to a neighbouring LH2 or into the ring of lower-energy LH1 B875 BChls surrounding the RC (Sundström et al., 1999) (Figure 6). The final step involves the transfer of excited state energy to the P870 dimer of BChls in the RC, triggering photochemical charge separation. Funneling of excited state energy into the RC is therefore achieved by having red-shifted (low-energy) BChls closest to the RC.
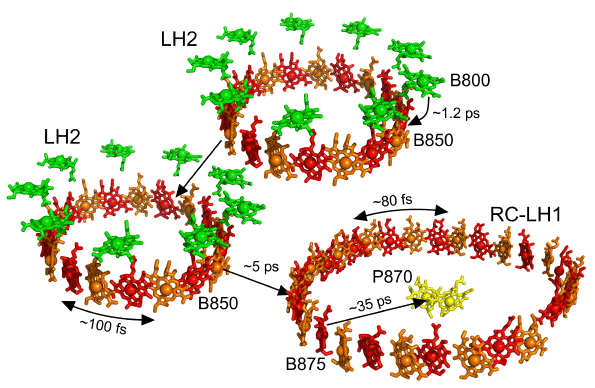
Figure 6. Energy transfer in the purple bacterial photosystem. B800 BChls of LH1 are shown in green, B850 BChls of LH2 and B875 BChls of LH1 are shown in alternating red and orange, and P870 BChls of the RC are shown in yellow. Lifetimes in picoseconds (ps) are given for the energy transfer events indicated by the arrows. Energy absorbed by antenna carotenoids is passed to neighbouring BChls. Double-headed arrows indicate femtosecond (fs) energy migration within the B850 or B875 pigment rings.
As the rate of energy transfer decreases with the sixth power of the distance between donor and acceptor (Sundström et al., 1999; Blankenship, 2002), light harvesting complexes need to be tightly-packed within the membrane to ensure that excited state energy is passed efficiently to the RC with minimal losses from emission. The excited state lifetime of BChl is of the order of a few nanoseconds, which means that energy absorbed by antenna pigments has to be funneled to the RC on a time scale of a few tens to hundreds of ps. Rates of particular steps in this process are summarised in Figure 6 (reviewed in Sundström et al., 1999). Energy transfer between weakly coupled BChls in adjacent LH complexes takes place on time scales of a few ps and, along with
B800
 B850 energy transfer, can be described by the Förster mechanism (non-radiative resonance energy transfer through coupling between two transition dipoles) (Sundström et al., 1999; Blankenship, 2002). Within the B850 and B875 rings, where the BChls are strongly coupled, the excitation is delocalized over the whole ring with extremely fast fs "hopping times" between adjacent BChls, and this process is better described using an exciton approach involving delocalized electronic transitions (Sundström et al., 1999; Blankenship, 2002).
B850 energy transfer, can be described by the Förster mechanism (non-radiative resonance energy transfer through coupling between two transition dipoles) (Sundström et al., 1999; Blankenship, 2002). Within the B850 and B875 rings, where the BChls are strongly coupled, the excitation is delocalized over the whole ring with extremely fast fs "hopping times" between adjacent BChls, and this process is better described using an exciton approach involving delocalized electronic transitions (Sundström et al., 1999; Blankenship, 2002).
The slowest step is the transfer of the excited state from the B875 BChls to the P870 BChl dimer (referred to as 'trapping'), which, due to the relatively large distance, takes place in around 30-50 ps. This arrangement, where the charge-separating Chls or BChls of the RC are separated from the light-harvesting Chls or BChls of the antenna by an exclusion zone formed by the protein scaffold, is also a feature of the structure of both PS1 and PS2 of oxygenic photosynthesis. This architecture appears to have two principal functions, to ensure that unproductive back transfer of excitation energy (detrapping) is slow compared to productive charge-separation (which takes place in a few ps), and to ensure that the efficiency of membrane-spanning charge separation is not interfered with by unwanted electron transfer reactions between RC and antenna BChls.
The process of photochemical charge separation is described in detail in the related module by Yocum on RCs. A summary of the reactions catalysed by the Rba. sphaeroides RC is shown in Figure 4B. Acquisition of excited state energy converts P870 into a sufficiently powerful reductant to be able to donate an electron to the adjacent BA BChl. This electron is subsequently passed in three steps to the quinone at the QB site near the cytoplasmic side of the membrane, the photooxidised P870 (P870+) located near the periplasmic face of the membrane being re-reduced (in Rba. sphaeroides) by a water-soluble c-type cytochrome
(cyt c2). A second light-induced charge separation results in double reduction of the QB quinone, delivery of the second electron being accompanied by the uptake of two protons from the cytoplasm to form ubiquinol [dihydroquinone (QH2)]. The immediate products of light absorption, energy funnelling and charge separation are therefore one QH2, which dissociates into the intra-membrane phase, and two oxidised cyt c2 (cyt c23+) which can diffuse in the periplasmic space. These mobile products are then used as substrates by the bc1 (Figure 5) (Hunte et al., 2003; Crofts, 2004).
Oxidation of QH2 takes place at a site (Qo) near the periplasmic side of the membrane and is a bifurcated reaction, one electron being used to reduce cyt c2 by passage through the so-called high-potential chain formed by the Rieske Fe-S protein and cyt c1 (Figure 5), accompanied by the release of a proton. A notable feature of this reaction is a large scale change in the conformation of the Rieske protein, that moves the Fe-S centre away from the Qo site and towards the cyt c1 heme. This movement prevents the second electron from being passed to the Rieske Fe-S centre. Instead, accompanied by release of the second proton, the second electron is passed back across the membrane via two b-type hemes to a second quinone reductase site (Qi). Oxidation of a second QH2 at the Qo site results in the reduction of a second cyt c2 via the high potential chain, and double reduction and protonation of the quinone at the Qi site. As the sites for reduction/protonation of quinone in both the RC and bc1 are located on the cytoplasmic side of the membrane, and the site for quinol oxidation in the latter is located on the periplasmic side, light-powered cyclic electron flow in this system is coupled to the translocation of protons from the cytoplasmic compartment into the periplasm. Figure 7 summarises the movement of quinone and cyt c2 between binding sites on the RC and bc1, and the proton translocation that is powered by sunlight.
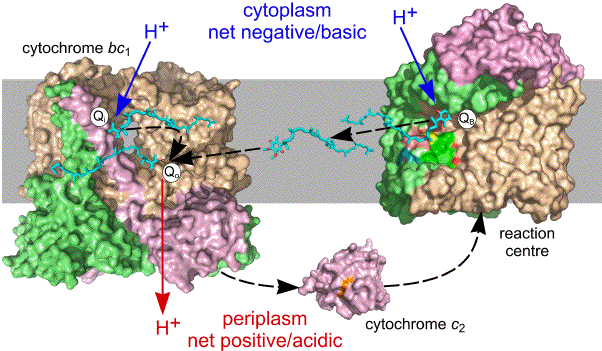
Figure 7. Light driven cyclic electron transfer and proton pumping. The RC acts in partnership with the bc1 to translocate protons across the photosynthetic membrane, transducing sunlight into the energy of the protonmotive force. Dashed black arrows show the movement of reducing equivalents between the RC and bc1, or between the cytoplasmic and periplasmic side sides of the bc1, by the mobile carriers ubiquinol and cyt c2. Blue arrows show sites for the uptake of protons from the cytoplasm, and red arrows the site of proton release into the periplasm. The migration of electrons internal to the RC and bc1 is not shown. The view of the bc1 dimer shows the Qi site of one monomer, and the Qo site of the second monomer
Summaries of this process from the points of view of redox potential and free energy are shown in Figure 8. Transformation of the redox potential of P870 through light absorption (Figure 8A) triggers a cascade of redox reactions, as electrons flow via the QB site into the intra-membrane
Q-pool, through the high potential chain of the bc1 , and through the cyt c2 pool to re-reduce P870+. Figure 8B shows operation of the RC from the standpoint of free energy. The additional energy of the initially-formed P870* excited state (~1.45 eV for an 870 nm photon) is progressively lost as charge is separated through the protein. However, part of this energy is preserved in the form of the energy of the pmf formed through the proton translocation that is coupled to electron flow. This pmf is then used to power a variety of energy-requiring reactions, including ATP synthesis, active transport, motion of the bacterial flagellum, and so on.
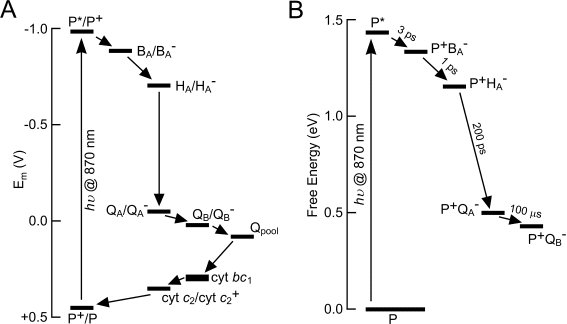
Figure 8. Redox potential and energetics of electron transfer in the Rba. sphaeroides photosystem.
(A) Formation of the excited state through light absorption transforms the redox potential of P870 (abbreviated to P in this diagram), triggering reduction of BA and subsequent electron transfer. "cyt bc1" refers to the high-potential chain in the bc1 complex. Redox couples are written with the notation reactant/product.
(B) Free energy of charge separation in the RC.
5. Photosystem Diversity
The outline description given in the last section is based on the photosystem from Rba. sphaeroides, which has been the main workhorse for structural and functional studies. The photosystems from other species of purple bacteria operate on the same principles, but the details vary from organism to organism. So, for example, the first RC to yield a high resolution X-ray crystal structure, that from Blc. viridis, has an additional subunit comprising an extra-membrane tetra-heme cytochrome that is attached to the periplasmic side of the complex (Deisenhofer et al., 1995). One of the hemes of this cytochrome subunit acts as the initial reductant of the photo-oxidised primary electron donor in this RC, termed P960+, the water-soluble c-type cytochrome delivering electrons from the bc1 to this tetra-heme cytochrome subunit, and so only indirectly reducing P960+. This RC contains BChl b rather than BChl a, and has menaquinone in the QA quinone site.
Perhaps the greatest variety comes in the type and organization of light-harvesting complexes present in purple bacteria. It has been known for many years that many species contain one or more types of peripheral light harvesting complex, the levels of which are affected by environmental factors such as light intensity or oxygen levels. In other species, including Blc. viridis and Rsp. rubrum, this peripheral antenna is absent, the light harvesting function being the sole responsibility of the LH1 antenna that surrounds each RC.
A full understanding of the process of photosynthetic energy transduction in purple bacteria not only requires detailed information on the structure and mechanism of individual components, but also an appreciation of how these are put together to form a fully functioning photosynthetic membrane. In recent years the technique of atomic force microscopy (AFM) has provided detailed information on this aspect of the photosystem, the distinctive ring-like topography of light harvesting complexes proving particularly amenable to imaging and interpretation (for reviews see Scheuring, 2006; Sturgis et al., 2008). In Blc. viridis, which lacks peripheral antenna complexes, the RC-LH1 complexes form very regular arrays, each unit in the array comprising a central RC surrounded by a complete ring of LH1 pigment-protein (comprised of 16 pairs of
 and ß polypeptides, see above).
and ß polypeptides, see above).
In Rsp. photometricum, the RC-LH1 complex also has a closed ring of 16
 /ß pairs, but the presence of LH2 means that the membrane is less ordered, with individual RC-LH1 complexes separated by variable amounts of smaller rings attributable to the peripheral LH2 complex. In Rps. palustris, the photosynthetic membrane is also disordered in this way, and in agreement with the low resolution X-ray data described above, the ring of LH1 surrounding each Rps. palustris RC is not complete, comprising only 15
/ß pairs, but the presence of LH2 means that the membrane is less ordered, with individual RC-LH1 complexes separated by variable amounts of smaller rings attributable to the peripheral LH2 complex. In Rps. palustris, the photosynthetic membrane is also disordered in this way, and in agreement with the low resolution X-ray data described above, the ring of LH1 surrounding each Rps. palustris RC is not complete, comprising only 15  /ß pairs. In Rba. blasticus, two forms of the RC-LH1 complex are apparent, a minor monomeric form comprising an RC surrounded by an incomplete ring of LH1 pigment-protein, and a major dimeric form in which two RCs are surrounded by a S-shaped antenna, both with 13 LH1
/ß pairs. In Rba. blasticus, two forms of the RC-LH1 complex are apparent, a minor monomeric form comprising an RC surrounded by an incomplete ring of LH1 pigment-protein, and a major dimeric form in which two RCs are surrounded by a S-shaped antenna, both with 13 LH1 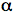 /ß pairs per RC. This dimeric arrangement was first reported for the Rba. sphaeroides RC-LH1 complex, and in this species RC-LH1 dimers are arranged in linear arrays several complexes in length, and surrounded by LH2 complexes (Bahatyrova et al., 2004). Although AFM has been successfully used to characterize the organization of RCs and LH complexes in photosynthetic membranes from a variety of purple bacteria, the location of the bc1 and ATP synthase in these membranes has not been established (Scheuring, 2006; Sturgis et al., 2008). However, the information from AFM and kinetic spectroscopy has been used to build spectacular models of the photosynthetic membrane (e.g., see Sener et al., 2007), which include speculations on the location of bc1 and ATP synthase.
/ß pairs per RC. This dimeric arrangement was first reported for the Rba. sphaeroides RC-LH1 complex, and in this species RC-LH1 dimers are arranged in linear arrays several complexes in length, and surrounded by LH2 complexes (Bahatyrova et al., 2004). Although AFM has been successfully used to characterize the organization of RCs and LH complexes in photosynthetic membranes from a variety of purple bacteria, the location of the bc1 and ATP synthase in these membranes has not been established (Scheuring, 2006; Sturgis et al., 2008). However, the information from AFM and kinetic spectroscopy has been used to build spectacular models of the photosynthetic membrane (e.g., see Sener et al., 2007), which include speculations on the location of bc1 and ATP synthase.
6. Metabolic Diversity
The mechanistic or physiological significance of observed variations in the organization of the photosynthetic membrane is not understood, but it may be linked, at least in part, to the metabolic flexibility displayed by this group of bacteria. The species displaying the greatest flexibility, including Rba. sphaeroides and the closely related Rba. capsulatus, are capable of both photoautotrophy (carry out photosynthesis to acquire energy and fix carbon dioxide), and photoheterotrophy (photosynthesis requiring a source of carbon other than carbon dioxide), and can also grow in the dark through aerobic respiration, or anaerobic respiration with a variety of terminal electron acceptors. Fermentative growth is also well documented, as is chemolithotrophy (deriving energy from the oxidation of inorganic compounds) with hydrogen or thiosulphate as electron donors. Most purple photosynthetic bacteria can fix N2 to form NH3 and H2, and some can carry out denitrification of nitrate to N2. Some species are obligate anaerobes, but others can tolerate high levels of oxygen, although in species such as Rba. sphaeroides expression of the photosynthetic apparatus is down-regulated by oxygen. In another group, termed aerobic anoxygenic phototrophs, expression of the photosynthetic apparatus takes place only in the presence of oxygen. These bacteria are widespread with a number of intriguing characteristics, including low levels of RCs and LH complexes, repression of BChl synthesis by light, and high levels of carotenoid.
A major factor in the prominent use of Rba. sphaeroides as a model organism for studying purple bacterial photosynthesis is its metabolic flexibility. In addition to growing in the light, this species will grow in the dark in the presence of a suitable source of carbon and electrons, such as succinate or malate for example, and a suitable electron acceptor, such as oxygen. Although the expression of the photosynthetic apparatus is repressed at very high levels of oxygen, a fully-functional photosystem is assembled at moderate-to-low oxygen tensions, although it is not required for growth. As a result, it is possible to introduce potentially lethal mutations into the RC and LH complexes of the photosystem, and study the structural and functional consequences of these in proteins or membranes prepared from cells grown in the absence of light. Such experiments are not possible in obligate phototrophs, where impairment of the photosynthetic apparatus can lead to cell death, and incubation under illuminated conditions can give rise to reversion or suppression mutations.
7. Photosynthesis in Other Anoxygenic Photosynthetic Bacteria
Although by far the most heavily studied, the purple phototrophs are only one of several groups of bacteria that use BChl proteins to exploit sunlight as an energy source. The green non-sulphur bacteria (also called green filamentous bacteria), such as Chloroflexus (Cf.) aurantiacus, have a photosystem similar to that of purple photosynthetic bacteria (Frigaard and Bryant, 2004). The RC of Cf. aurantiacus is similar to that of Rba. sphaeroides, with an intramembrane heterodimer of L and M polypeptides that scaffold the electron transfer cofactors. However, this RC lacks the H-polypeptide, both quinones are menaquinone, and the bacteriochlorin cofactors comprise three BChls and three BPhes. The RC is associated with a B808-866 antenna complex that has spectroscopic similarities to the purple bacterial LH2 antenna, but shows sequence similarities to the polypeptides of the purple bacterial LH1. In addition to possessing an intra-membrane light harvesting system, green non-sulphur bacteria possess an extensive light harvesting system called a chlorosome, which is attached to the cytoplasmic face of the photosynthetic membrane (Blankenship, 2002). This comprises an aggregate of up to 10,000 molecules of BChl c, with smaller amounts of protein, lipid and carotenoids, and the chlorosome transfers energy to the intra-membrane antenna via a set of baseplate proteins, and thence to the RC.
The RCs from purple bacteria and green filamentous bacteria are both classed as quinone-pheophytin type, or type-II RCs, along with the PS2 RC from oxygenic photosynthesis. The remaining anoxygenic photosynthetic bacteria, the green sulphur bacteria and heliobacteria, contain an Fe-S type or type-I RC similar to the PS1 RC from oxygenic photosynthesis (Heathcote et al., 2002). Again the reader is referred to the module by Yocum on RCs for a description of the PS1 RC; the key differences with the type-II RCs discussed thus far, is that the membrane-spanning electron transfer chain terminates in Fe-S centres rather than dissociable quinones, and within the RC pigment-protein the central electron transfer domain is flanked by two additional symmetrical domains that house antenna BChls or Chls and carotenoids. In green sulphur bacteria, such as Chlorobium limicola, the light harvesting function carried out by the intra-membrane antenna regions of the RC (Hauska et al., 2001) is again augmented by an extra-membrane chlorosome antenna comprising largely of BChls c, d, and e (Blankenship, 2002). This is also connected to the intramembrane antenna via baseplate proteins, but the green sulphur bacteria also contain the Fenna-Matthews-Olson (FMO) protein that participates in energy flow from the chlorosome to the intra-membrane components. The FMO protein is water-soluble, and was the first chlorophyll protein to be structurally characterized to a high resolution by X-ray crystallography (Olson, 2004).
The final group of anoxygenic photosynthetic bacteria are the heliobacteria. These also have a type-I RC, with a central electron transfer domain and two antenna domains, and unlike other photosynthetic bacteria this RC contains BChl g as a minor component (Hirozo, 2007). Heliobacteria do not have additional intra- or extra-membrane antenna complexes. A feature of the heliobacterial RC, shared with the type-I RC from green sulphur bacteria, is that it is formed from a homodimer of a single polypeptide, rather than the L/M heterodimer seen in purple bacteria (or the D1/D2 and PsaA/PsaB heterodimers seen in PS1 and PS2). Another feature established in recent is years is that, in common with PS1, both branches of membrane-spanning cofactors are used to transfer electrons across the membrane during photochemical charge separation. In the type-II RCs described above, the two quinones have discrete functional roles, the QA quinone acting as a one electron relay but the QB quinone being specialized for accumulation of two electrons and undergoing double protonation in order to generate a quinol. However, in the type-I RCs the quinone cofactors are both non-dissociable, and on reduction pass the electron on to an Fe-S centre located on the symmetry axis. As a result, in principle, either branch of cofactors could be used to catalyse membrane-spanning electron transfer, and findings in recent years have shown that this is indeed the case (see Heathcote et al., 2002, for a review).
8. Summary
Anoxygenic photosynthetic bacteria are a widespread and metabolically-diverse group of organisms that make a significant contribution to energy input into the biosphere from sunlight. In addition to their native contribution, these bacteria have been at the forefront of laboratory research into the mechanism of photosynthetic energy transduction by natural systems. A small number of species of purple phototrophs, in particular, have provided major insights into a number of key biochemical and biophysical processes, including the molecular details of solar energy conversion (Hu et al., 2002; Cogdell et al., 2004; Jones, 2009), the nature of biological electron transfer (Moser et al., 1992), the mechanistic basis of chemiosmotic energy transduction (Crofts et al., 2004a, b), and the structural biology of integral membrane proteins (Deisenhofer and Michel, 1989).
9. General Reading
Blankenship,R.E. (2002) Molecular Mechanisms of Photosynthesis. Blackwell Science Ltd., Oxford, United Kingdom.
Hunter, C.N., Daldal, F., Thurnauer, M.C., and Beatty, J.T. (eds) (2008) The Purple Phototrophic Bacteria. Advances in Photosynthesis and Respiration, 28, Springer, Dordrecht, The Netherlands.
10. References
Allen, J.P., Feher, G., Yeates, T.O., Komiya, H. and Rees, D.C. (1987) Structure of the reaction center from Rhodobacter sphaeroides R-26 - the cofactors, Proc. Natl. Acad. Sci. USA 84, 5730-5734.
Bahatyrova, S., Frese, R.N., Siebert, C.A., Olsen, J.D., van der Werf, K.O., van Grondelle, R., Niederman, R.A., Bullough, P.A., Otto, C. and Hunter C.N. (2004) The native architecture of a photosynthetic membrane. Nature 430, 1058-1062.
Cogdell, R.J., Gardiner, A.T., Roszak, A.W., Law, C.J., Southall, J. and Isaacs, N.W. (2004) Rings, ellipses and horseshoes: how purple bacteria harvest solar energy. Photosynth. Res. 81, 207-214.
Cogdell, R.J., Gall, A. and Köhler, J. (2006) The architecture and function of the light-harvesting apparatus of purple bacteria: from single molecules to in vivo membranes. Quart. Rev. Biophys. 39, 227-324.
Crofts, A.R. (2004a) The cytochrome bc1 complex: Function in the context of structure. Ann. Rev. Physiol. 66, 689-733.
Crofts, A.R. (2004b) The Q-cycle: a personal perspective. Photosynth. Res. 80, 223-243.
Deisenhofer, J. and Michel, H. (1989) The photosynthetic reaction center from the purple bacterium Rhodopseudomonas viridis. EMBO J. 8, 2149-2170.
Deisenhofer, J., Epp, O., Sinning, I. and Michel, H. (1995) Crystallographic refinement at 2.3-angstrom resolution and refined model of the photosynthetic reaction-center from Rhodopseudomonas viridis. J. Mol. Biol. 246, 429-457.
Esser, L., Elberry, M., Zhou, F., Yu, C.A., Yu, L. and Xia, D. (2008) Inhibitor-complexed structures of the cytochrome bc1 from the photosynthetic bacterium Rhodobacter sphaeroides. J. Biol. Chem. 283, 2846-2857
Frigaard, N.U. and Bryant, D. (2004) Seeing green bacteria in a new light: genomics-enabled studies of the photosynthetic apparatus in green sulfur bacteria and filamentous anoxygenic phototrophic bacteria. Arch. Micro. 182, 265-276
Hauska, G., T. Schoedl, H. Remigy and G. Tsiotis (2001) The reaction center of green sulfur bacteria. Biochim. Biophys. Acta 1507, 260-277.
Heathcote, P., Fyfe, P.K. and Jones, M.R. (2002) Reaction centres: the structure and evolution of biological solar power. Trends in Biochemical Sciences 27, 79-87
Hirozo, O. (2007) Type 1 reaction center of photosynthetic heliobacteria. Photochem. Photobiol., 83, 177-186.
Holden-Dye, K., Crouch, L.I. and Jones, M.R. (2008) Structure, function and interactions of the PufX protein. Biochim. Biophys. Acta - Bioenergetics 1777, 613-630.
Hu, X., Damjanovic, A., Ritz, T. and Schulten, K. (1998) Architecture and mechanism of the light-harvesting apparatus of purple bacteria, Proc. Natl. Acad. Sci. USA 95, 5935-5941.
Hu, X.C., Ritz, T., Damjanovic, A., Autenrieth, F. Schulten, K. (2002) Photosynthetic apparatus of purple bacteria. Quart. Rev. Biophys. 35, 1-62.
Hunte, C., Palsdottir, H. and Trumpower, B.L. (2003) Protonmotive pathways and mechanisms in the cytochrome bc1 complex. FEBS Letts. 545, 39-46.
Jones, M.R. (2009) The petite purple photosynthetic powerpack. Biochem. Soc. Trans. 37, 400-407.
Koepke, J., Hu, X.C., Muenke, C., Schulten, K., Michel, H. (1996) The crystal structure of the light-harvesting complex II (B800-850) from Rhodospirillum molischianum. Structure 4, 581-597.
Law, C.J., Roszak, A.W., Southall, J., Gardiner, A.T., Isaacs, N.W., Cogdell, R.J. (2004) The structure and function of bacterial light-harvesting complexes. Mol. Memb. Biol. 21, 183-191.
McDermott, G., Prince, S.M., Freer, A.A., Hawthornthwaite-Lawless, A., Papiz, M.Z. Cogdell, R.J., Isaacs, N.W. (1995) Crystal-structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature 374, 517-521.
Moser, C.C., Keske, J.M., Warncke, K., Farid, R.S. and Dutton, P. L. (1992) Nature of biological electron transfer, Nature 355, 796-802.
Olson, J.M. (2004) The FMO protein. Photosynth. Res., 80, 181-187
Papiz, M.Z., Prince, S.M., Howard, T., Cogdell, R.J. and Isaacs N.W. (2003) The structure and thermal motion of the B800-850 LH2 complex from Rps. acidophila at 2.0 Å resolution and 100 K : new structural features and functionally relevant motions. J. Mol. Biol. 326, 1523-1538
Potter, J.A., Fyfe, P.K., Frolov, D., Wakeham, M.C., van Grondelle, R., Robert, B. and Jones, M.R. (2005) Strong effects of an individual water molecule on the rate of primary charge separation in the Rhodobacter sphaeroides reaction centre. J. Biol. Chem., 280, 27155-27164.
Roszak, A.W., Howard, T.D., Southall, J., Gardiner, A.T., Law, C.J., Isaacs, N.W. and Cogdell, R.J. (2003) Crystal structure of the RC-LH1 core complex from Rhodopseudomonas palustris. Science 302, 1969-1972.
Scheuring, S. (2006) AFM studies of the supramolecular assembly of bacterial photosynthetic core-complexes. Curr. Op. Chem. Biol. 10, 387-393.
Sener, M.K., Olsen, J.D., Hunter, C.N. and Schulten, K. (2007) Atomic-level structural and functional model of a bacterial photosynthetic membrane vesicle. Proc. Natl. Acad. Sci. USA 104, 15723-15728.
Sturgis, J.N. and Niederman, R.A. (2008) Atomic force microscopy reveals multiple patterns of antenna organization in purple bacteria: implications for energy transduction mechanisms and membrane modeling. Photosynth. Res. 95, 269-278.
Sundström, V., Pullerits, T. and van Grondelle, R. (1999) Photosynthetic light-harvesting: reconciling dynamics and structure of purple bacterial LH2 reveals function of photosynthetic unit. J. Phys. Chem. B 103, 2327-2346.
Sundström, V. (2008) Femtobiology. Annu. Rev. Phys. Chem. 59, 53-77.
10/08/09