PHOTOMORPHOGENIC RESPONSES OF PLANTS TO UV-B RADIATION
Gareth I. Jenkins
Institute of Molecular, Cell and Systems Biology, College of Medical, Veterinary and Life Sciences, Bower Building, University of Glasgow, Glasgow G12 8QQ, UK
Gareth.Jenkins@Glasgow.ac.uk
Light acts as the driving force for plant growth through the process of photosynthesis but, importantly, light also provides environmental cues that regulate plant development throughout the lifecycle. The regulation of development by light, termed photomorphogenesis, is mediated by several different photoreceptors that detect particular parameters of the radiation environment, and initiate photomorphogenic responses via their associated signal transduction pathways (Jiao et al., 2007). Much research has been devoted to determining the functions of different photoreceptors in photomorphogenesis, and the identity of their downstream signaling components. It is well established that the phytochrome photoreceptors detect principally red and far-red light, whereas the cryptochromes, phototropins and zeitlupe family proteins detect UV-A and blue light (Li and Yang, 2007; Christie, 2007; Franklin and Quail, 2010). Low levels of UV-B light (280-315 nm) also elicit photomorphogenic responses in plants (Frohnmeyer and Staiger, 2003; Ulm and Nagy, 2005; Jenkins, 2009; Jiang et al., 2012; Heijde and Ulm, 2012), but until recently the identity of the UV-B photoreceptor remained a mystery. This article will summarise photomorphogenic responses to UV-B radiation, and their regulation by the UV-B photoreceptor UVR8.
Photomorphogenic Responses to UV-B Radiation. It has been known for many years that UV-B exposure modifies plant growth and biochemical content (Klein 1978). Building on previous observations, research in the 1970’s characterised several photomorphogenic responses to UV-B light that could not be explained by the action of known photoreceptors. These responses were initiated by low doses of UV-B, and hence were not caused by damage to DNA or other molecules (Wellmann, 1976; 1983). Whereas the action spectrum for DNA damage peaks at approximately 260 nm, the action spectra of the photomorphogenic UV-B responses had maxima around 295-300 nm (Wellmann, 1976). Subsequent studies of various photomorphogenic UV-B responses have revealed similar action spectra (Ensminger, 1993; Jenkins, 2009; Jiang et al., 2012).
One of the first identified and best characterized photomorphogenic responses to UV-B is the biosynthesis of particular flavonoid secondary metabolites. Flavonoid biosynthesis is stimulated by several light qualities and also by non-light signals (Winkel-Shirley, 2002; Jenkins, 2008), so this response is not specific to UV-B exposure. Moreover, there are considerable differences both between species and between flavonoid products in the extent of regulation by UV-B. Nonetheless, the stimulation of flavonoid biosynthesis by low doses of UV-B is observed in a wide range of species and is a vital protective response. Particular flavonoids, notably flavonols, strongly absorb UV-B wavelengths and hence their biosynthesis and accumulation in epidermal layers of the shoot provides a sunscreen, helping to protect plants from potential damage caused by elevated levels of UV-B (Klein, 1978; Jordan, 1996; Rozema et al., 1997; Ryan et al., 2001). Plant genotypes that are impaired in flavonoid biosynthesis are more susceptible to damage by UV-B (Li et al., 1993; Stapleton and Walbot, 1994; Ryan et al., 2001).
The stimulation of flavonoid biosynthesis by UV-B is primarily due to the regulation of gene expression (Hahlbrock and Scheel, 1989). UV-B treatment of plants stimulates the transcription of genes encoding the first enzyme in the flavonoid biosynthesis pathway, Chalcone Synthase (CHS) (Chappell & Hahlbrock, 1984; Jenkins et al., 2001), and enzymes that act downstream in the pathway (Hahlbrock and Scheel, 1989; Hartmann et al., 2005). Research has identified DNA sequence elements in the promoter regions of flavonoid biosynthesis genes that are required for induction by UV-B, and transcription factor proteins that interact with these elements to mediate the response (Schulze-Lefert et al., 1989; Hartmann et al., 2005). CHS expression is stimulated by very low doses of UV-B, and is observed within a few hours of UV-B treatment (Figure 1A). Moreover, transcriptome analyses and assays of specific transcripts show that numerous other genes are also regulated by low doses of UV-B via a photomorphogenic pathway (Ulm et al., 2004, Brown et al., 2005; Brown and Jenkins, 2008; Favory et al., 2009).
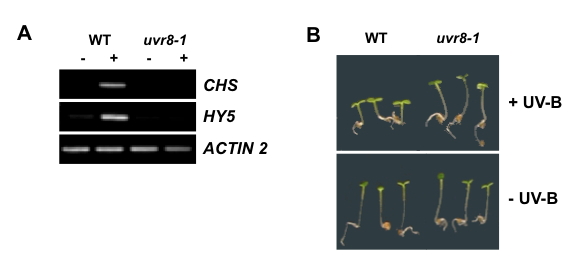
Figure 1. Photomorphogenic Responses to UV-B Radiation.
A. Levels of CHS and HY5 (Elongated Hypocotyl 5) transcripts in wild-type (Ler) and uvr8-1 mutant plants exposed (+), or not (-), to 3 µmol m-2s-1 broadband UV-B for 4 hours. Levels of CHS and HY5 transcripts in RNA samples were assayed by RT-PCR (Reverse Transcriptase-Polymerase Chain Reaction) relative to transcripts of the control ACTIN2 gene, essentially as described by Brown and Jenkins (2008).
B. Hypocotyl lengths of wild-type (Ler) and uvr8-1 seedlings grown for 4 days in 1.5 µmol m-2s-1 fluorescent white light supplemented (+), or not (-), with 1.5 µmol m-2s-1 narrowband UV-B (method described in Cloix et al., 2012). [Data of M. Heilmann and G.I. Jenkins]
In addition to flavonoid biosynthesis, photomorphogenic UV-B perception regulates several aspects of morphogenesis and development (Tevini and Teramura 1989; Jansen 2002, Caldwell et al., 2003; Frohnmeyer and Staiger, 2003; Jenkins 2009). It is commonly observed that UV-B exposure reduces stem extension and leaf expansion, but additional effects have been reported in various species; for instance, in Arabidopsis, UV-B stimulates cotyledon opening (Boccalandro et al., 2001), modifies inflorescence development (Hectors et al., 2007) and suppresses root extension (Tong et al., 2008). An important issue is to distinguish the regulatory effects of UV-B from those caused by UV-B damage to cells, which may impair cell division and expansion. For instance, relatively high doses of UV-B inhibit hypocotyl extension growth in cucumber (Shinkle et al., 2004) and Arabidopsis (Kim et al., 1998; Boccalandro et al., 2001), most likely through molecular damage, whereas lower doses inhibit growth through photomorphogenic effects. In tomato, the suppression of hypocotyl growth by low fluence UV-B was maximal around 300 nm, indicating that the response was not mediated by DNA damage (Ballaré et al., 1995). Moreover, experiments with Arabidopsis mutants indicate that the photomorphogenic suppression of hypocotyl extension by UV-B (shown in Figure 1B) is not mediated either by DNA damage, cryptochromes or phytochromes A or B (Boccalandro et al., 2001; Suesslin and Frohnmeyer, 2003).
Attempts to Understand UV-B Photomorphogenic Responses.
Although the above research demonstrated unequivocally that UV-B light initiated photomorphogenic responses in plants, little progress was made over several decades in elucidating the mechanisms of UV-B perception. Nevertheless, research succeeded in further characterizing several photomorphogenic UV-B responses, and in eliminating several candidate perception mechanisms. Thus, experiments indicated that photomorphogenic responses to UV-B were not mediated either by the known photoreceptors, DNA damage signaling, wound or defence signaling pathways (Jenkins et al., 2001; Jenkins, 2009). Further experiments using pharmacological reagents in cell cultures provided evidence that UV-B signaling was distinct from phytochrome and cryptochrome signaling, and suggested that cellular calcium and redox activity may be involved in UV-B responses (Christie and Jenkins,1996; Frohnmeyer et al., 1997; Long and Jenkins, 1998). There was speculation about the nature of the putative UV-B photoreceptor and whether it might contain, for example, flavin or pterin chromophores (Ensminger and Schäfer 1992; Ballaré et al., 1995), but no UV-B photoreceptor was identified. Hence, it was clear that a different approach was needed to discover the mechanisms of photomorphogenic UV-B photoreception and signaling.
Considerable progress had been made in understanding responses mediated by other photoreceptors through the application of a genetic approach in Arabidopsis, and it was anticipated that a similar approach would facilitate progress in UV-B research. The difficulty was in choosing the best response to screen for mutants altered specifically in UV-B perception. A screen for impaired hypocotyl growth suppression in response to UV-B exposure produced the uli3 (UV-B light insensitive 3) mutant (Suesslin and Frohnmeyer, 2003), but no convincing evidence has been presented that the corresponding protein has a specific involvement in UV-B signaling. Screens for mutants altered in sensitivity to UV-B provided information principally on components involved in DNA repair or sunscreen biosynthesis (Jenkins and Brown, 2007). However, one mutant hypersensitive to UV-B had a novel phenotype, and is now known to identify a key component in UV-B perception: UV Resistance Locus 8 (UVR8) (Kliebenstein et al., 2002). The uvr8 mutant was found to have impaired expression of the CHS gene in response to UV-B (Figure 1A), and hence reduced levels of flavonoid sunscreen pigments. In addition, uvr8 displayed increased expression of the PR-1 gene, which is induced by a non-photomorphogenic UV-B pathway, and is indicative of stress. The UVR8 gene was found to encode a protein with sequence similarity to human Regulator of Chromosome Condensation 1 (RCC1), a protein involved in regulation of cell division and transport of components into and out of the nucleus. Subsequent research showed that UVR8 is not a functional orthologue of RCC1 (Brown et al., 2005), although it is related structurally, as discussed further below.
UVR8: A UV-B Photoreceptor
Physiological Significance. Brown et al. (2005) isolated further uvr8 alleles by screening for impaired CHS promoter activity in response to UV-B exposure and, importantly, showed that UVR8 acts specifically to mediate photomorphogenic responses to UV-B. Furthermore, transcriptome analyses of uvr8 mutant plants in comparison to wild-type revealed that UVR8 regulates the expression of at least 100 genes in response to UV-B treatment (Brown et al., 2005, Favory et al., 2009). Although the functions of some of these genes are unclear, many are involved in protecting plants against the potential damage caused by exposure to elevated levels of UV-B. For example, UVR8 regulates the expression of genes encoding flavonoid biosynthesis enzymes, explaining why uvr8 mutant plants have much reduced levels of UV-absorbing sunscreen compounds (Kliebenstein et al., 2002). In addition, UVR8 regulates genes concerned with minimizing oxidative stress and repairing DNA damage following UV-B exposure. Furthermore, several genes regulated by UVR8 encode chloroplast proteins, consistent with evidence that UVR8 helps to maintain photosynthetic competence (Davey et al., 2012). Hence, the altered gene expression of uvr8 mutant plants explains their intolerance of elevated levels of UV-B.
UVR8 also regulates aspects of morphogenesis. Arabidopsis uvr8 mutant plants are impaired in the UV-B induced suppression of hypocotyl extension (Figure 1B; Favory et al., 2009; Cloix et al., 2012; O’Hara and Jenkins, 2012), and in the regulation of leaf expansion (Wargent et al., 2009). It is likely that these morphogenic defects are due to impaired gene expression, but it is not known which genes are involved.
Most action spectra of photomorphogenic UV-B responses have peaks in the 280 nm to 300 nm range, although some extend to 310 nm (Jenkins, 2009; Jiang et al., 2012). The action spectrum for induction of gene expression by UVR8 indicates that the photoreceptor is most effective at 280 nm, with significant action at 290 and 300 nm (Brown et al., 2009). However, since the 280 nm wavelength does not reach the surface of the earth, the longer wavelength action of UVR8 will be the most physiologically relevant. The UVR8 amino acid sequence is highly conserved among plant species, including in non-vascular plants such as mosses and green algae (Rizzini et al., 2011). Although studies to date have been limited to just a few species, it is likely that the protein evolved to help early photosynthetic plants survive exposure to UV-B wavelengths in sunlight.
Transcriptional Regulation. Although UVR8 acts by regulating transcription of a set of target genes involved in photomorphogenic responses, UVR8 itself is not a transcription factor protein and is therefore likely to act by regulating the abundance, activity and/or recruitment of transcription factors for target genes. An important observation is that UVR8 regulates the expression of two transcription factors that function downstream of UVR8 to initiate photomorphogenic responses to UV-B, namely HY5 and HY5 Homolog (HYH) (Brown et al., 2005; Brown and Jenkins, 2008; Favory et al., 2009). UV-B treatment stimulates a rapid, transient increase in transcript levels of these two genes, which is impaired in uvr8 mutant plants (see Figure 1A for HY5 expression). Transcriptome analysis combined with expression studies of several genes indicates that HY5 has a key role in mediating the UV-B regulation of many UVR8 target genes (Ulm et al., 2004; Brown et al., 2005; Brown and Jenkins, 2008; Stracke et al., 2010) whereas HYH has a secondary role, acting redundantly with HY5 in the regulation of some genes (Brown and Jenkins, 2008). Furthermore, HY5 has a key role in conferring protection against UV-B, since hy5 mutant plants are highly sensitive to UV-B, similar to uvr8 plants.
It is not clear how UVR8 regulates transcription. UVR8 binds to chromatin via histones (Brown et al., 2005; Cloix and Jenkins, 2008), suggesting the basis of a mechanism for UVR8 function in regulating transcription. Moreover, UVR8 associates with chromatin containing the HY5 gene, and additional target genes, but not all UVR8 regulated genes. It is unclear why UVR8 associates with chromatin containing some genes it regulates and not others, so at present the molecular basis and significance of the interaction with chromatin is not understood. Nevertheless, it seems likely that UVR8 interacts with other proteins associated with chromatin to promote remodeling and the recruitment or activation of transcription factors that stimulate transcription of its target genes.
Structure and Photoreception. UVR8 is a member of the relatively large family of 7-bladed β-propeller proteins (Kliebenstein et al., 2002; Christie et al., 2012; Wu et al., 2012), which also includes RCC1. Rizzini et al. (2011) discovered that UVR8 exists as a homodimer prior to UV-B exposure, and that exposure to low doses of UV-B causes dissociation of the dimer into monomers (Figure 2).
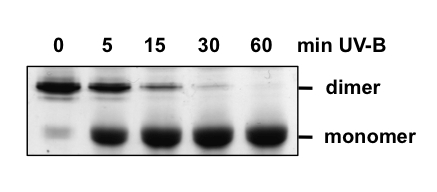
Figure 2. UV-B Induces Monomerisation of UVR8. Purified UVR8 protein exposed to 1.5 µmol m-2s-1 narrowband UV-B for the times indicated, and separated into dimer and monomer by SDS-polyacrylamide gel electrophoresis with unboiled samples. [Modified from Christie et al., 2012]
Monomerisation is observed with the purified protein (Christie et al., 2012; Wu et al., 2012), in plants and in heterologous systems (Rizzini et al., 2011; Cloix et al., 2012). A crystal structure has been obtained for dimeric UVR8 lacking approximately 40 amino acids from the C-terminus (Figure 3A; Christie et al., 2012; Wu et al., 2012). The structure reveals that the dimer is maintained by salt-bridges formed between specific charged amino acids located on the interacting surfaces of two monomers (the dimer interface). In particular, positively charged amino acids, principally arginines, interact with negatively charged aspartate and glutamate residues. Mutation of specific charged amino acids prevents the formation of key salt bridges, causing the mutant UVR8 proteins to become constitutively monomeric (Christie et al., 2012; Wu et al., 2012). The working hypothesis for UVR8 photoreceptor action is that UV-B photoreception leads to neutralization of these key salt bridges causing monomerisation. However, the intramolecular processes that couple photoreception to charge neutralisation are not yet known.
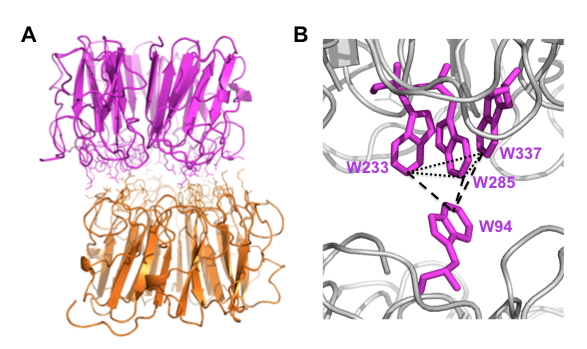
Figure 3. Structure of UVR8.
A. Crystal structure of dimeric UVR8 lacking approximately 40 C-terminal amino acids (Christie et al., 2012), highlighting amino acids at the dimer interface.
B. Pyramidal arrangement of excitonically coupled tryptophans across the dimer interface.
Characterisation of purified UVR8 shows that it does not possess a bound organic molecule to act as a UV-B absorbing chromophore for the photoreceptor. This feature is in contrast to all other known photoreceptors, whose wavelength specificity is determined by the nature of their bound chromophores. The ability of UVR8 to act as a UV-B photoreceptor is therefore determined by the ability of intrinsic amino acids to function as UV-B absorbing chromophores. Aromatic amino acids, most notably tryptophan, are effective in UV-B absorption. UVR8 possesses 14 tryptophans in its primary sequence, but studies with UVR8 both in vitro and in vivo reveal that only a few specific tryptophans have a role in photoreception. Three tryptophans of UVR8 (W233, W285 and W337), located at the dimer interface are sufficiently close that their electronic orbitals overlap. These excitonically coupled ‘triad’ tryptophans are in close proximity to W94 on the opposite monomer, forming a pyramidal arrangement across the dimer interface (Christie et al., 2012; Figure 3B). Two such ‘pyramids’ are present in each dimer. Absorption of UV-B by one or more pyramid tryptophans results in a loss of exciton coupling and causes disruption of the salt-bridges, and hence monomerisation. W285 and W233 are most strongly implicated in UV-B absorption by UVR8 in studies of the purified protein (Christie et al., 2012; Wu et al., 2012). Experiments in yeast (Rizzini et al., 2011) and in transgenic plants (Christie et al., 2012; O’Hara and Jenkins, 2012) support this conclusion, but highlight the key role of W285 as the principal UV-B chromophore of UVR8. W285 is adjacent to a key salt-bridging arginine, R286, but it is not clear how photoreception leads to neutralization of the cross-dimer salt bridges.
An important feature of UVR8 is that monomers reassociate to form dimers in darkness following UV-B exposure. This provides a mechanism for regeneration of the active photoreceptor. However, the rate of dimer regeneration is much faster in intact plants than it is in vitro with the purified protein. There is evidence that the interaction of proteins with the C-terminal region of UVR8 promotes reassociation to form the dimer (Heilmann and Jenkins, 2013; Heijde and Ulm, 2013).
Signal Transduction. It is important to understand how photoreception by UVR8 initiates the signal transduction processes that lead to transcriptional responses. One process associated with signal transduction is the accumulation of UVR8 in the nucleus (Kaiserli and Jenkins, 2007). The localization of UVR8 fused to Green Fluorescent Protein (GFP) can be monitored by fluorescence microscopy. UVR8 is present in both the cytosol and nucleus, but exposure to low fluence rates of UV-B stimulates the rapid accumulation of GFP-UVR8 in the nucleus. However, nuclear localization of UVR8 is required, but is not sufficient for its function in regulating target gene expression (Kaiserli and Jenkins, 2007).
Constitutively Photomorphogenic 1 (COP1) is the only protein known to act with UVR8 to promote photomorphogenic UV-B responses (Oravecz et al., 2006). The Arabidopsis cop1-4 mutant is impaired in the UV-B induction of many genes regulated by UVR8, including HY5, indicating that UVR8 and COP1 act together. This positive regulatory function of COP1 contrasts with its well-established role as a negative regulator of photomorphogenesis in dark-grown seedlings (Lau and Deng, 2012); in this context it acts as an E3 ubiquitin ligase, initiating the proteolytic destruction of positive regulators of photomorphogenesis, including the HY5 protein (Osterlund et al., 2000).
UV-B exposure stimulates a physical interaction between UVR8 and COP1 (Favory et al., 2009; Figure 4). This interaction is seen not only in plants but also when the two proteins are expressed in heterologous cells, such as yeast (Rizzini et al., 2011; Cloix et al., 2012). A 27-amino acid region towards the C-terminus of UVR8 (termed C27) interacts with the WD40 domain of COP1. Deletion of this region from UVR8 prevents both the interaction with COP1 and the ability of UVR8 to initiate responses in vivo (Cloix et al., 2012).
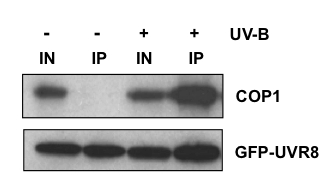
Figure 4. UV-B Stimulates Interaction Between UVR8 and COP1. Co-immunoprecipitation assay using uvr8-1 plants transformed with a GFP-UVR8 fusion. Whole cell extracts were obtained from plants treated (+) or not (-) with 3 µmol m-2s-1 narrowband UV-B for 3 hours. GFP-UVR8 was immunoprecipitated and the immunoprecipitate assayed for the presence of COP1 using an antibody. Aliquots of ‘input’ protein samples used in the assay (IN), and immunoprecipitated protein (IP) were fractionated by SDS-polyacrylamide gel electrophoresis and western blots were probed with anti-COP1 and anti-GFP antibodies. [Modified from Cloix et al., 2012]
A possible model is that the C27 region is not available to interact with COP1 in dimeric wild-type UVR8, but becomes exposed following UV-B photoreception and monomerisation (Figure 5). However, the situation appears to be more complex because some UVR8 mutant proteins interact with COP1 even in the absence of UV-B, although this interaction is not sufficient to initiate signal transduction (O’Hara and Jenkins, 2012). Hence, UV-B photoreception may induce additional changes in UVR8 that are needed to initiate signaling, such as a conformational change. A key functional consequence of the interaction between UVR8 and COP1 is the regulation of target gene expression, although it is not clear how association between the proteins actually modulates transcription at the level of chromatin. In addition, it is likely that the interaction impairs the E3 ubiquitin ligase activity of COP1, preventing it acting as a negative regulator of photomorphogenesis.
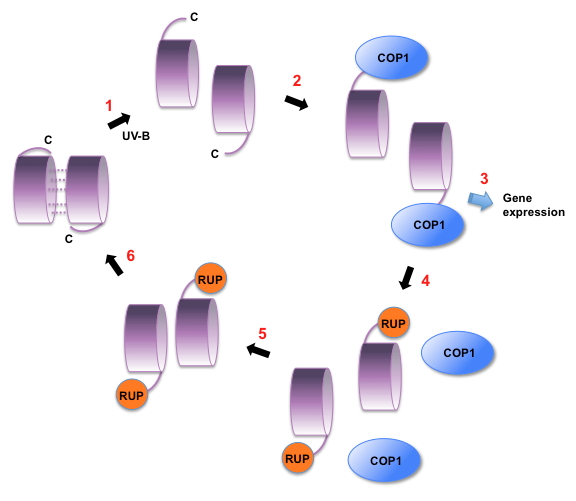
Figure 5. Model of UVR8 Regulation.
1. UV-B photoreception by dimeric UVR8 forms monomers. It is likely that a conformational change makes the C-terminus of the protein available for interaction with COP1. However, the location of the C-terminus is not known.
2. COP1 binds to the C27 region of UVR8. It is not known whether other regions of UVR8 are involved in the interaction.
3. UVR8 together with COP1 initiates transcription of target genes.
4. Among the genes induced are those encoding the RUP proteins, which negatively regulate UVR8. RUP1 and RUP2 bind to the C27 region of UVR8 and may act by displacing COP1.
5. The RUP proteins are involved in facilitating re-association of monomers to form the dimer.
6. The dimer is regenerated for photoreception.
UVR8 interacts with two further proteins, Rexpresser of UV-B Photomorphogenesis (RUP) 1 and 2 (Gruber et al., 2010; also known as Early Flowering By Overexpression 1 and 2; Wang et al., 2011). The rup mutants have elevated responses to UV-B indicating that RUP1 and RUP2 negatively regulate UVR8 mediated responses in vivo (Gruber et al., 2010). These RUP proteins interact with the C27 region of UVR8 (Cloix et al., 2012), and so it is possible that they act by impairing the interaction with COP1 (see Figure 5). In addition, there is evidence that the RUP proteins promote the re-association of UVR8 monomers, which regenerates the active dimeric photoreceptor (Heijde and Ulm, 2013). A further negative regulator of UVR8 signaling is the transcription factor BBX24, although its regulation and mechanism of action in UV-B responses is quite complex, and not fully understood (Jiang et al., 2012). Hence, further research is needed to investigate the interactions of UVR8 with other proteins and to establish their functional significance.
Conclusions
It is now well established that UV-B initiates photomorphogenic responses in plants. Some of these responses have evolved to protect plants against damage by UV-B, and therefore promote survival in sunlight. Recent research has identified UVR8 as a UV-B photoreceptor that mediates photomorphogenic responses. UVR8 is different to other known photoreceptors in that instead of using a prosthetic chromophore for light absorption, it uses several of its tryptophan amino acids as chromophores for UV-B detection. Photoreception causes UVR8 monomerisation and initiates signaling through interaction with COP1. However, much remains to be learned about the molecular mechanism of UVR8 photoreception, and how changes to the UVR8 protein initiate signaling. Furthermore, very little is known about how UVR8 regulates the transcription of its target genes.
References
Ballaré CL, Barnes PW, Flint SD (1995) Inhibition of hypocotyl elongation by ultraviolet-B radiation in de-etiolating tomato seedlings. 1. The photoreceptor. Physiol. Plant 93: 584-592.
Boccalandro HE, Mazza CA, Mazzella MA, Casal JJ, Ballaré CL (2001) Ultraviolet B radiation enhances a phytochrome-B-mediated photomorphogenic response in Arabidopsis. Plant Physiol. 126: 780-8.
Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI (2005) A UV-B-specific signaling component orchestrates plant UV protection. Proc. Natl. Acad. Sci. USA 102: 18225-18230.
Brown BA, Headland LR, Jenkins GI (2009) UV-B action spectrum for UVR8-mediated HY5 transcript accumulation in Arabidopsis. Photochem. Photobiol. 85: 1147-1155.
Brown BA, Jenkins GI (2008) UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol. 146: 576-588.
Caldwell MM, Ballaré CL, Bornman JF, Flint SD, Björn LO, Teramura AH, Kulandaivelu G, Tevini M (2003) Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochem. Photobiol. Sci. 2: 29-38.
Chappell J, Hahlbrock K (1984) Transcription of plant defence genes in response to UV light or fungal elicitor. Nature 311: 76-78.
Christie JM (2007) Phototropin blue-light receptors. Ann. Rev. Plant Biol. 58: 21-45.
Christie JM, Arvai AS, Baxter KJ, Heilmann M, Pratt AJ, O’Hara A, Kelly SM, Hothorn M, Smith BO, Hitomi K, Jenkins GI, Getzoff ED (2012) Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335: 1492-1496.
Christie JM, Jenkins GI (1996) Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 8: 1555-1567.
Cloix C, Jenkins GI (2008) Interaction of the Arabidopsis UV-B-specific signaling component UVR8 with chromatin. Mol. Plant 1: 118-128.
Cloix C, Kaiserli K, Heilmann M, Baxter KJ, Brown BA, O’Hara A, Smith BO, Christie JM, Jenkins GI (2012) The C-terminal region of the UV-B photoreceptor UVR8 initiates signaling through interaction with COP1. Proc. Natl. Acad. Sci. USA 109: 16366-16370.
Davey MP, Susanti NI, Wargent JJ, Findlay JE, Quick WP, Paul ND, Jenkins GI (2012) The UV-B photoreceptor UVR8 promotes photosynthetic efficiency in Arabidopsis thaliana exposed to elevated levels of UV-B. Photosynth. Res. 114: 121-131.
Ensminger PA (1993) Control of development in plants and fungi by far-UV radiation. Physiol. Plant. 88: 501-508.
Ensminger PA, Schäfer E (1992) Blue and ultraviolet-B light photoreceptors in parsley cells. Photochem. Photobiol. 55: 437-447.
Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, Seidlitz HK, Nagy F, Ulm R (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 28: 591-601.
Franklin KA, Quail PH (2010) Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61: 11-24.
Frohnmeyer H, Bowler C, Schafer E (1997) Evidence for some signal transduction elements involved in UV-light-dependent responses in parsley protoplasts. J. Exp. Bot. 48: 739-50.
Frohnmeyer H, Staiger D (2003) Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 133: 1420-1428.
Gruber H, Heijde M, Heller W, Albert A, Seidlitz HK, Ulm R (2010) Negative feedback regulation of UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Proc. Natl. Acad. Sci. USA 107: 20132-20137.
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40: 347-369.
Hartmann U, Sagasser M, Mehrtens F, Stracke R, Weisshaar B (2005) Differential combinatorial interactions of cis-acting elements recognized by R2R3-MYB, BZIP, and BHLH factors congtrol light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes. Plant Mol. Biol. 57: 155-171.
Hectors K, Prinsen E, De Coen W, Jansen MAK, Guisez Y (2007) Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol. 175: 255-270.
Heijde M, Ulm R (2012) UV-B photoreceptor-mediated signaling in plants. Trends in Plant Science 17: 230-237.
Heijde M, Ulm R (2013) Reversion of the Arabidopsis UV-B photoreceptor UVR8 to the homodimeric ground state. Proc. Natl. Acad. Sci. USA 110: 1113-1118.
Heilmann M, Jenkins GI (2013) Rapid reversion from monomer to dimer regenerates the ultraviolet-B photoreceptor UV RESISTANCE LOCUS8 in intact Arabidopsis plants. Plant Physiol. 161: 547-555.
Jansen MAK (2002) Ultraviolet-B radiation effects on plants: induction of morphogenic responses. Physiol. Plant. 116: 423-29.
Jenkins GI (2008) Environmental regulation of flavonoid biosynthesis. In: Health Benefits of Organic Food: Effects of the Environment. Givens I, Baxter S, Minihane AM, Shaw E (eds.) CABI, Wallingford, UK; pp.240-262.
Jenkins GI (2009) Signal transduction in responses to UV-B radiation. Ann. Rev. Plant Biol. 60: 407-31.
Jenkins GI, Brown BA (2007) UV-B perception and signal transduction. In: Light and Plant Development. Whitelam GC, Halliday KJ (eds.) Blackwell Publishing, Oxford; pp.155-182.
Jenkins GI, Long JC, Wade HK, Shenton MR, Bibikova TN (2001) UV and blue light signalling: pathways regulating chalcone synthase gene expression in Arabidopsis. New Phytol. 151: 121-131.
Jiang L, Wang Y, Björn LO, He J-X, Li, S-S (2012) Sensing of UV-B radiation by plants. Plant Signaling & Behavior 7: 1-5.
Jiao Y, Lau OS, Deng XW (2007) Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8: 217-230.
Jordan BR (1996) The effects of ultraviolet-B radiation on plants: a molecular perspective. Adv. Bot. Res. 22: 97-162.
Kaiserli E, Jenkins GI (2007) UV-B promotes rapid nuclear translocation of the UV-B-specific signaling component UVR8 and activates its function in the nucleus. Plant Cell 19: 2662-2673.
Kim BC, Tennessen DJ, Last RL (1998) UV-B-induced photomorphogenesis in Arabidopsis thaliana. Plant J. 15: 667-674.
Klein RM (1978) Plants and near-ultraviolet radiation. Bot. Rev. 44: 1-127.
Kliebenstein DJ, Lim JE, Landry LG, Last RL (2002) Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human Regulator of Chromatin Condensation 1. Plant Physiol. 130: 234-243.
Lau OS, Deng XW (2005) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 17: 584-593.
Li JY, Oulee TM, Raba R, Amundson RG, Last RL (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5: 171-179.
Li QH, Yang HQ (2007) Cryptochrome signaling in plants. Photochem. Photobiol. 83: 94-101.
Long JC, Jenkins GI (1998) Involvement of plasma membrane redox activity and calcium homeostasis in the UV-B and UV-A/blue light induction of gene expression in Arabidopsis. Plant Cell 10: 2077-2086.
O’Hara A, Jenkins GI (2012) In vivo function of tryptophans in the Arabidopsis UV-B photoreceptor UVR8. Plant Cell 24: 3755-3766.
Oravecz A, Baumann A, Máté Z, Brzezinska A, Molinier J, Oakeley EJ, Ádám É, Schäfer E, Nagy F, Ulm R (2006) CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell 18: 1975-1990.
Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462-466.
Rizzini L, Favory J-J, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, Ulm R (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science, 332: 103-106.
Rozema J, van de Staaij J, Björn LO, Caldwell M (1997) UV-B as an environmental factor in plant life: Stress and regulation. Trends Ecol. Evolution 12: 22-28.
Ryan KG, Swinny EE, Winefield C, Markham KR (2001) Flavonoids and UV photoprotection in Arabidopsis mutants. Z. Naturforsch. C 56, 745-754.
Schulze-Lefert P, Becker-André M., Schulz W, Hahlbrock K, Dangl JL (1989) Functional architecture of the light-responsive chalcone synthase promoter from parsley. Plant Cell 1: 707-714.
Shinkle JR, Atkins AK, Humphrey EE, Rodgers CW, Wheeler SL, Barnes PW (2004) Growth and morphological responses to different UV wavebands in cucumber (Cucumis sativum) and other dicotyledonous seedlings. Physiol. Plant 120: 240-248.
Stapleton AE, Walbot V (1994) Flavonoids can protect maize DNA from the induction of ultraviolet-radiation damage. Plant Physiol. 105: 881-889.
Stracke R, Favory J-J, Gruber H, Bartelniewoehner L, Bartels S, Binkert M, Funk M, Weisshaar B, Ulm R (2010) The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Env. 33, 88-103.
Suesslin C, Frohnmeyer H (2003) An Arabidopsis mutant defective in UV-B light-mediated responses. Plant J. 33: 591-601.
Tevini M, Teramura AH (1989) UV-B effects on terrestrial plants. Photochem. Photobiol. 50: 479-487.
Tong H, Leasure CD, Hou X, Yuen G, Briggs W, He ZH (2008) Role of root UV-B sensing in Arabidopsis early seedling development. Proc. Natl. Acad. Sci. USA 105: 21039-21044.
Ulm R, Baumann A, Oravecz A, Mate Z, Adam E, Oakeley EJ, Schäfer E, Nagy F (2004) Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 1397-1402.
Ulm R, Nagy F (2005) Signalling and gene regulation in response to ultraviolet light. Curr. Opinion Plant Biol. 8: 477-482.
Wang W, Yang D, Feldman K (2011) EFO1 and EFO2, encoding putative WD-domain proteins, have overlapping and distinct roles in the regulation of vegetative development and flowering of Arabidopsis. J. Exp. Bot. 62: 1077-1088.
Wargent JJ, Gegas VC, Jenkins GI, Doonan JH, Paul ND (2009) UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation. New Phytol. 183: 315-326.
Wellmann E (1976) Specific ultraviolet effects in plant morphogenesis. Photochem. Photobiol. 24, 659-60.
Wellman E (1983) UV radiation in photomorphogenesis. In: Encyclopedia of Plant Physiology New Series. Shropshire W Jr., Mohr H (eds.) Springer, Berlin; Vol. 16B pp.745-756.
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr. Opinion Plant Biol. 5: 218-223.
Wu D, Hu Q, Yan Z, Chen W, Yan C, Huang X, Zhang J, Yang P, Deng H, Wang J, Deng X, Shi Y (2012) Structural basis of ultraviolet-B perception by UVR8. Nature 484: 214-219.
06/24/13