PHOTOMORPHOGENESIS in NATURAL LIGHT ENVIRONMENTS
Keara A. Franklin1 and James R. Shinkle2
1School of Biological Sciences, University of Bristol, Woodland Road, Bristol, BS8 1UG, UK
kerry.franklin@bristol.ac.uk
2Department of Biology, Trinity University,
San Antonio, Texas 78212-7200, USA
jshinkle@trinity.edu
Introduction
Photoautotrophic higher plants are dependent upon light for their survival. The light environment varies continuously, with both spatial and temporal fluctuations occurring daily. As sessile organisms that cannot choose their surroundings, plants need to modify their growth and development in order to optimize their utilization of ambient light. Plants monitor the quantity, quality, periodicity and direction of light, and use this information to modulate all aspects of development, from seed germination and seedling establishment, to mature plant architecture and the onset of reproduction. Such developmental plasticity is conferred by specialized families of information-transducing photoreceptors. In higher plants, there are three principle families of such photoreceptors; the red/far-red (R/FR) light-absorbing phytochromes, and the UV-A/blue light-absorbing cryptochromes and phototropins. In Arabidopsis thaliana, five discrete apophytochrome-encoding genes, PHYA-PHYE, have been isolated and sequenced (Mathews and Sharrock, 1997), whereas the cryptochrome and phototropin families each comprise two members (Briggs and Huala, 1999; Cashmore et al., 1999). A third cryptochrome may have been identified (Selby and Sancar, 2006), but the one function ascribed to this protein, so far, is that of a photolyase, not a photoreceptor.
This article is mainly focused on the role of the phytochromes in the regulation of photomorphogenesis in natural light environments, in particular the shade avoidance syndrome. This syndrome is a suite of developmental responses initiated by the reflected/transmitted light signals generated by neighboring vegetation, and represents perhaps the most extensively studied example of adaptive phenotypic plasticity in higher plants.
Natural Light Environments
All visible light energy on earth is provided by the sun, with the solar radiation that reaches the earth's atmosphere having a spectral photon distribution similar to that of a black-body radiator at a surface temperature of 5,800°K. This radiation is significantly attenuated within the atmosphere. Short-wavelength radiation is selectively absorbed by the ozone layer, whilst oxygen and water vapor display strong absorption bands in the longer wavelength regions of the visible spectrum and into the FR (wavelengths beyond 700 nm region) (Smith, 1975). A typical spectral energy distribution of incident daylight is shown in Figure 1.
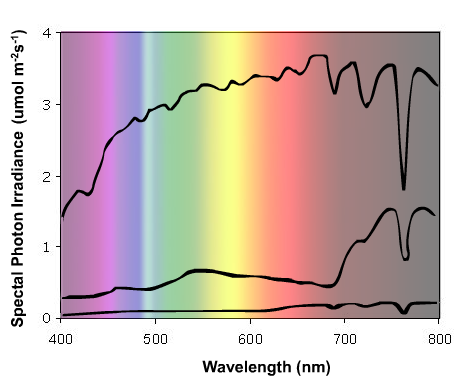
Figure 1. The spectral energy distribution of incident solar radiation on a clear day under the following conditions: Upper curve = midday, Middle curve = midday sun filtered through a canopy of mustard seedlings, Lower curve = dusk. Note the small change in R/FR ratio (655-665 nm / 725-735 nm) between midday and dusk compared to the much larger change caused by light absorption by leaf tissue.
Providing the sun is more than 10° above the horizon, the spectral energy distribution of daylight is fairly constant, and is only modestly influenced by cloud cover or haze. A useful parameter to describe the natural light environment is the ratio of photon irradiance in the R, to that in the FR (R:FR ratio). This parameter is directly related to the properties of phytochrome and is often precisely defined as follows:
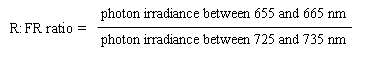
The R:FR ratio of daylight is around 1.15 and varies little with weather conditions or the time of year (Smith 1982).
The progression of the sun across the sky leads to daily fluctuations in the spectral quality of daylight. At solar elevations of less than 10° (as seen at dawn and dusk), the increasing path length through the earth's atmosphere leads to enhanced absorption and scattering, as well as refraction of the solar beam by the atmosphere, resulting in the preferential enhancement of longer wavelengths. This, together with an increased contribution of scattered skylight, leads to a twilight spectrum that is relatively enriched in the blue and the FR regions, but relatively poor in the orange-red regions. Thus, the onset of sunrise and dusk are both associated with a significant drop in R:FR ratio from about 1.15 to about 0.7-0.8. There is preliminary evidence that at high latitudes where twilight duration is extended, the change in light quality is used to provide seasonal information (Linkosalo and Lechowicz, 2006).
The spectral energy distribution of daylight is dramatically altered by vegetation. The photosynthetic pigments, chlorophylls and carotenoids, absorb light over most of the visible spectrum, although some of the green light is reflected or transmitted. This is, of course, why leaves appear green to our eyes. Radiation in the FR region is very poorly absorbed by vegetation, and consequently, the light that is transmitted through or reflected from vegetation displays a significantly reduced R:FR ratio, compared with daylight (Figures 1 and 2). Reported R:FR ratios underneath vegetational canopies are typically in the range 0.09 - 0.7 (Smith, 1982). Such reductions in R:FR can be detected by neighboring plants as a change in the relative proportions of the red vs. the far red forms of phytochrome (Pr and Pfr, respectively), thus providing a unique and unambiguous signal that potential competitors are nearby. Furthermore, the extent of the reduction in R:FR ratio is quantitatively related to the density and proximity of the neighboring vegetation (Smith and Whitelam, 1997).
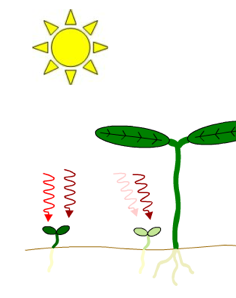
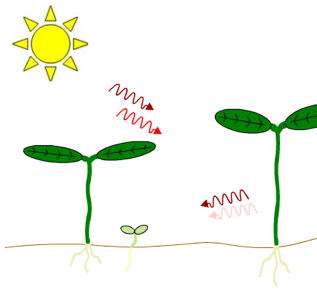
Figure 2. Effects of neighboring plants on the ratio of red light to far-red light perceived by a plant in a canopy. Both light filtered through leaves and reflected off nearby stems or leaves will be enriched in far-red light relative to direct sunlight. Left Figure: Far-red light passes through the leaf canopy much more readily than red light. Thus, plants growing under the canopy receive light with a lower red/far-red ratio than plants in direct sunlight. Note the difference in greening. Right Figure: The spectrum of light reflected by a stem differs from the spectrum of the incident light because some of the light that strikes the stem is absorbed by the stem.
The only other circumstance where the natural radiation environment shows significant alteration in R:FR ratio is underwater. Water displays strong absorption bands in the FR (at about 730 nm) and the in the infra-red region. As daylight passes through water, there is selective attenuation of FR such that with increasing depth there is an increase in R:FR ratio. In addition, many natural waters contain organic material, leading to significant attenuation of the blue and R regions.
Photomorphogenesis in Natural Light Environments
Germination. Seed germination depends on many environmental circumstances, including position in the soil profile, soil disturbance and extent of canopy cover. In many species, these responses are mediated, in part, by phytochrome perception of the light environment (Botto et al., 1996; Shinomura et al., 1996). Imbibed seeds and etiolated seedlings display three different modes of phytochrome action, characterised by different fluence rate response curves, R/FR reversibility, and fluence rate dependency. These are the very low fluence response (VLFR), the low fluence response (LFR) and the high irradiance response (HIR) (Furuya and Schäfer, 1996). The first two categories of responses can be initiated by brief (on the order of seconds) pulses of light, while the third requires continuous irradiation over long (on the order of hours) periods. The germination of many seeds is dependent upon an exposure to light, and in most cases germination displays classical R/FR reversibility characteristic of the LFR mode of phytochrome action. [As a caution to the reader, note that FR means either "far-red" or "fluence response" depending on the context.] For Arabidopsis seeds, the R/FR-reversible promotion of germination has been shown to be regulated by phyB and phyE (Hennig et al., 2002). It can be speculated that this response allows buried seeds to detect proximity to the soil surface or for seeds on the surface, to detect canopy gaps.
Many seeds that have been imbibed in darkness remain dormant but acquire extreme sensitivity to light typical of the VLFR mode of phytochrome action. The VLFR is mediated by phyA. It is estimated that these sensitized seeds would be induced to germinate following exposure to only a few milliseconds of daylight (Smith, 1982). Such sensitivity enables seeds to take opportunistic advantage of very brief soil disturbances (see Awakened by a Flash of Sunlight). In some seeds that are capable of germinating in the dark, germination is inhibited by exposure to prolonged FR, probably reflecting the far-red high irradiance response (FR-HIR) mode of phytochrome action. The FR-HIR is mediated by phyA, and the inhibition of seed germination in FR-rich light environments may be ecologically relevant as a means of delaying the germination of seeds situated under chlorophyllous vegetation or leaf litter (Smith and Whitelam 1990). There is also a blue-light induced HIR, which appears to also be mediated by phyA (Chun et al., 2001; Neff and Chory, 1998; Weller et al., 2001), but the ecological significance of this response is uncertain.
Germination at different temperatures can alter which Phy contributes to the regulation of the process. Heschel et al. (2008) found that PhyB was more important in seeds that were not imbibed at cold temperatures
(4° C) and that PhyD played a major regulatory role for seeds imbibed at high temperature (31° C). Further, this paper reported that under cetain conditions, both PhyA and PhyD were active in suppressing rather than promoting germination. The area of temperature effects on Phy action is a growing area of inquiry (Franklin, 2009a).
Seedling De-etiolation. Dark-grown seedlings follow a developmental program (termed skotomorphogenesis) displaying a characteristic phenotype: elongated stems, closed and unexpanded cotyledons, and non-photosynthetic etioplasts, which are chloroplasts in their developmental precursor state (see Figure 3). Upon transfer to light, axis growth is inhibited, cotyledons are expanded, and etioplasts undergo rapid morphological changes to form photosynthetically active chloroplasts. The inhibition of axis elongation and other aspects of photomorphogenesis under natural light environments involve the action of several photoreceptors. The contributions of individual photoreceptors in regulating plant photomorphogenesis are studied in the laboratory using monochromatic light sources. The role of phyB in the red-light-regulation of hypocotyl inhibition is well documented (Koornneef et al., 1980; McCormac et al., 1993; Somers et al., 1991). Such inhibition is enhanced by co-actions with phyA (Reed et al., 1994). Cryptochromes also play a significant role in hypocotyl/stem elongation in response to blue/UV-A light (Ahmad and Cashmore, 1997). A role of phyA in blue light-sensing has been recorded in a variety of species (Chun et al., 2001; Neff and Chory, 1998; Weller et al., 2001). The phyA-mediated FR-HIR may also play an important role in seedling de-etiolation under natural canopy shade conditions. Although seedlings growing in the natural environment would never be exposed to prolonged FR, it has been shown that Arabidopsis phyA-deficient mutant seedlings grown under dense vegetational shade (a FR-rich light environment) display severely impaired de-etiolation and extreme hypocotyl elongation (Yanovsky et al 1995). A significant number of these phyA mutant seedlings failed to become established and died. Wild-type seedlings are less elongated under these conditions, presumably due the action of phyA in the FR-HIR.

Figure 3. Note the differences in growth between the Arabidopsis seedlings grown under different light conditions. Left: Dark-grown (etiolated) Arabidopsis seedling at 3 days. Right: White light-grown Arabidopsis seedling at 3 days displaying an inhibition of hypocotyl elongation and cotyledon development.
The development of etioplasts into chloroplasts is regulated by diverse light signals and photoreceptors. Etioplasts contain prolammellar bodies; paracrystalline structures composed largely of lipids and NADPH:protochlorophyllide oxidoreductase in its photosensitive ternary complex (Apel et al., 1980). Upon illumination, the prolammelar body disperses and initiates a series of defined processes which result in the development of the mature chloroplast. Chlorophyll is formed in the plastid from its precursor, 5-aminolevulinic acid (ALA), via the tetrapyrrole pathway (Beale, 1990). Control of the pathway involves complex interacting regulatory mechanisms with ALA formation providing the site of primary photocontrol (Kasemir, 1983). Reporter gene studies have revealed transcription of glutamyl-tRNA reductase, the first committed enzyme of ALA synthesis, to be light regulated through several phytochromes, subject to modification from plastid-induced signals (McCormac and Terry, 2002).
Proximity Perception. The ability to modulate both architecture and reproductive strategy in response to the perceived threat of shading remains one of the most radical adaptive strategies available to higher plants. Neighbor detection initiates avoidance responses in "shade intolerant" species, enabling plants to compete for light. Such responses include enhanced internode and petiole extension growth, increased apical dominance, retarded leaf development and an acceleration of flowering (Halliday et al., 1994; Smith and Whitelam, 1997). Figure 4 shows a typical shade avoidance response in mustard (Sinapis alba) and Arabidopsis grown under high and low R:FR ratios. These physiological adaptations are accompanied by changes in the distribution of assimilates between leaves, stems and roots (Keiller and Smith, 1989), and serve to elevate leaves to a better-lit stratum in the canopy, and promote seed set at a time when resources may be limiting. Plants respond predominantly to the reduction in R:FR ratio reflected from surrounding vegetation, and therefore initiate physiological responses before they are directly shaded (Ballare et al., 1990; see also Know Thy Neighbor Through Phytochrome). The ability to respond to the perceived threat of shading, and therefore execute architectural changes before canopy closure, is a crucial competitive strategy in rapidly growing populations.

Figure 4. Shade avoidance responses in Mustard (Sinapis alba). Under low red to far red ratio (R:FR), plants display stem and petiole elongation, altered leaf shape and early flowering.
Although phytochrome signal transduction and regulation of genes is beyond the scope of this treatise, a relatively complete model has emerged for how phytochromes change gene expression related to the increased stem elongation seen in shade avoidance. This model incorporates nuclear localized regulatory proteins known to interact with phytochromes (Phytochrome Interacting Factors, PIFs ; see Monte et al., 2007; Bae and Choi, 2008) and proteins previously identified as mediating responses to gibberellins, one of the families of plant growth regulators. This latter association is not surprising given the history of observations of apparent similarities between phytochrome and gibberellins mediated growth responses (Chory and Li, 1997) and the nuclear proteins involved, the DELLA proteins, are central to gibberellins regulation of stem elongation (Harberd, 2003). A direct interaction between a member of the PIF family associated with shade avoidance (PIF4) and the DELLA protein RGA has recently been demonstrated (DeLucas et al., 2008).
Figure 5 shows the proposed series of events changing gene expression as a daylight-grown plant (high R:FR ratio) is subject to the threat of vegetational shade (low R:FR ratio). The model is based on the following features of the proteins: 1) when Phys are converted to the Pfr form, they migrate to the nucleus. PhyA is degraded by the 26S proteosome quickly as Pfr. PhyB complexes with PIF4, and both are degraded. 2) PIF4 is a transcription factor that activates transcription of genes involved in increased elongation growth. 3) DELLA proteins bind PIF4 and prevent it from activating genes involved in increased elongation. 4) DELLA proteins accumulate in daylight conditions (Achard et al., 2007). Based on these features, under daylight PhyB in the Pfr form accumulates in the nucleus and interacts with PIF4. When both are degraded, this lowers the total amount of PIF4 in the nucleus. The rest is complexed by DELLA proteins, so that little is available to activate transcription. When a plant first encounters reductions in R:FR, PhyB reverts to the Pr form and releases PIF4. PIF4 is then available to activate genes for elongation growth. DELLA levels may also drop on withdrawal from high R:FR ratio light. Low R:FR ratio conditions also cause some increase in PhyA in the Pfr form reaching the nucleus, sufficient to complex some PIF4, and antagonize the PhyB mediated effect. As a plant continues in shade for a prolonged period gibberellins synthesis is induced by PhyB Pfr (Hisamatsu et al., 2005). Gibberellins bind to the receptor which is the protein product of the Gibberellin-Insensitive Dwarf1 (GID1) gene, and this causes degradation of DELLA proteins (Harberd et al., 2009), releasing even greater numbers of PIF4 proteins to activate transcription.
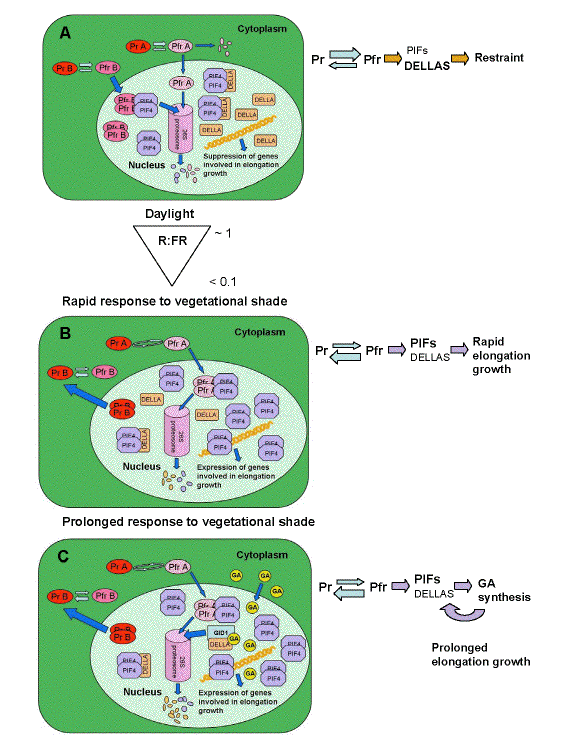
Figure 5. Hypothetical model depicting molecular mechanisms controlling red to far-red ratio (R:FR) mediated elongation growth. (a) In daylight PhyB exists predominantly in the Pfr form (PfrB). Phytochrome A Pfr is degraded both in the cytosol and in the nucleus. Following import into the nucleus PfrB binds PIF4 resulting in their degradation by the 26S proteosome. DELLA proteins bind remaining PIF4 preventing these molecules from activating transcription. Daylight also increases DELLA abundance leading to suppression of transcription of genes involved in elongation growth. (b) At the onset of low R:FR in vegetational shade, most PhyB is converted to the Pr from, causing it to disassociate from PIF4 and exit the nucleus. Somewhat counterbalancing the release of PIF4, the low R:FR ratio causes PhyA to cycle between Pr and Pfr forms, protecting it from degradation. Some of the PfrA reaches the nucleus and binds to PIF4, leading to degradation. DELLA proteins may decline in abundance because of absence of induction of synthesis, also causing PIF4 release. There is an overall increase in abundance of PIF4 available to activate transcription. (c) Prolonged exposure to vegatational shade permits feedback from the elongation related gene products, which include enzymes involved in gibberellin (GA) biosynthesis. Increased GA induces degradation of DELLA via the formation of the GA/GID1/DELLA complex that is directed to the proteasome resulting in further release of PIF4 (from Franklin, 2009b).
The involvement of phyB in mediating responses to R:FR ratio is widely documented in a variety of species (Devlin et al., 1992; Lopez-Juez et al., 1992; Reed et al., 1993; Somers et al., 1991), although the retention of shade avoidance responses in phyB null mutants indicated the involvement of additional phytochromes (Halliday et al., 1994; Robson et al., 1993; Whitelam and Smith, 1991). Subsequent investigations have revealed shade avoidance responses in Arabidopsis to be mediated solely by phyB, D and E acting together in a functionally redundant manner (Devlin et al., 1996, 1998, 1999). These represent the most recently evolved members of the phytochrome family, forming a distinct subgroup (Mathews and Sharrock, 1997). It can therefore be speculated that competition for light may have provided the selective pressure for the evolution of this subgroup (Devlin et al., 1998).
Ecological investigations using herbaceous plants have suggested that the elongated phenotypes induced by low R:FR confer high relative fitness in dense stands, but would prove disadvantageous in areas of sparse vegetation (Schmitt, 1997). Reallocation of resources towards elongation growth, in the absence of competition, may reduce overall fitness in addition to increasing the risk of mechanical damage to stems (Casal and Smith, 1989). Constitutive expression of a PHYA gene in transgenic tobacco was shown to result in the suppression of normal shade avoidance responses through persistence of the normally transient FR-HIR (McCormac et al., 1991,1992). When planted in dense stands, the ensuing inability of transgenic plants to elongate resulted in a decrease in fitness as measured by dry biomass (Robson et al., 1996; Schmitt et al., 1995). Conversely, when a constitutively elongated phyB-deficient mutant of Brassica rapa was grown in a low density mixed population, decreases in both dry biomass and number of reproductive structures were observed (Schmitt et al., 1995). The functional disadvantage of stem elongation in the absence of competition was further supported by observations showing elongated phyB-deficient mutants of cucumber to display increased mechanical damage when grown individually in the field (Casal et al., 1994; Schmitt, 1997).
Evolution of trees as well as herbs has resulted in species which possess the capacity to sense neighbours. The significance of shade avoidance in developing forests was demonstrated by the recent discovery that responses to reduced R:FR ratio in three species of tree were inversely proportional to the proximity signals generated by those species (Gilbert et al 2001). Early successional species therefore generated the smallest proximity signals, but reacted most strongly to them, whereas late successional species behaved oppositely.
Photoperiodic Perception. In many plant species, responses to light are influenced by the time of day in which the light is perceived. Sensitivity to the timing of light and darkness, termed photoperiodism, enables plants to adapt to seasonal changes in their surroundings. The ability to anticipate and consequently prevent the adverse effects of a particular seasonal environment confers considerable advantage to the plant (Thomas and Vince-Prue 1997). The concept of photoperiodism was first introduced by Garner and Allard (1920) who observed that flowering and many other responses in plants could be accelerated by either long or short days. Supporting evidence was later provided by Parker et al. (1946), and Borthwick et al. (1952). In photoperiodically-sensitive species, the onset of sexual or vegetative reproduction is governed by the relationship between the day-length received, and a threshold or "critical" day-length. The timing of reproduction often only occurs when days are sufficiently short (short-day plants; SDP) or long (long-day plants; LDP). Plants insensitive to photoperiodic induction are termed "day neutral". The measurement of daylength involves the integration of temporal information, provided by the circadian oscillator, with light/dark discrimination provided by specific photoreceptors. In the LDP Arabidopsis, flowering is accelerated under long day conditions. Here, the presence of light is perceived through the action of either cryptochrome 2 (cry2) or phyA (Yanovsky and Kay, 2002). The effects of these photoreceptors are, as in the case of shade avoidance, mediated through a cascade of gene-regulation events. One common final product is the graft transmissible protein product of the Flowering Locus T gene (FT) (Lagercranz 2009). The photoperiodic induction of tuberization in the SDP Solanum tuberosum ssp. Andigena has been shown to involve phyB in the production of a graft-transmissible inhibitor (Jackson et al., 1998). The identity of this inhibitor remains to be elucidated with some evidence implicating a role for a class of plant growth regulatory molecules called gibberellins (Jackson and Prat, 1996), but more recent work suggesting that a relative of FT is the mobile signal (Rodriguez-Falcon et al., 2006).
Conclusions
The phenotypic plasticity awarded by effective monitoring of the ambient light environment confers considerable selective advantage to plants growing in natural communities. The capacity to respond to perceived threat of shading promotes survival by enabling plants to overtop competing vegetation, and promotes seed maturation when supply of resources may be uncertain. Synchronization of flowering time to environmental cues, such as photoperiod, enhances survival through promoting seed maturation during favourable seasonal conditions, and limiting competition with neighbours for often limited resources. Such synchronization may also increase the chance of out-breeding and genetic recombination. The multiplicity of responses available to plants in response to environmental light signals results from functional divergence within the phytochrome family of photoreceptors. Redundancy between family members and co-actions with blue light sensing mechanisms increase sensitivity to environmental fluctuations and permit an array of developmental responses. Conditional modulation of phytochrome functional hierarchy by ambient temperature facilitates further diversification of phytochrome function. The majority of studies to date have, however, focused on a very limited number of plant species, requiring the future elucidation of photoreceptor mutants in a variety of species to broaden our understanding of adaptive plasticity in mixed communities in natural light environments.
Literature Cited
Achard P, Liao L, Jiang C, Desnos T, Bartlett,J, Fu X, and Harberd NP (2007) DELLAs contribute to plant photomorphogenesis. Plant Physiology 143: 1163-1172.
Ahmad M and Cashmore AR (1997) The blue light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J. 11: 421-427.
Apel K, Santel H-J, Redlinger TE and Falk H (1980) The protochlorophyllide holochrome of barley (Hordeum vulgare L.). Isolation and characterisation of the NADPH:protochlorophyllide oxidoreductase. Eur. J. Biochem. 111: 251-258.
Bae G, and Choi G (2008) Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 59: 281-311.
Ballaré CL, Scopel AL and Sánchez RA (1991) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247: 329-332.
Beale SI (1990) Biosynthesis of the tetrapyrrole pigment precursor d-aminolevulinic acid from glutamate. Plant Physiol. 93: 1273-1279.
Botto JF, Sanchez RA, Whitelam GC and Casal JJ (1996) Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 110: 439-444.
Borthwick HA, Hendricks SB and Parker MW (1952) The reaction controlling floral initiation. Proc. Natl. Acad. Sci. USA 38: 929-934.
Briggs WR and Huala E (1999) Blue-light photoreceptors in higher plants. Ann Rev Cell Dev Biol 15: 33-62.
Casal JJ, Ballaré CL, Tourn M and Sánchez RA (1994) Anatomy, growth and survival of a long-hypocotyl mutant of Cucumis sativis deficient in phytochrome B. Annals Bot. 73: 569-575.
Casal JJ and Smith H (1989) The function, action and adaptive significance of phytochrome in light-grown plants. Plant Cell Environ. 12: 855-862.
Cashmore AR, Jarillo JA, Wu YJ and Liu D (1999) Cryptochromes: blue light receptors for plants and animals. Science 284: 760-765.
Chory J, Li J (1997) Gibberellins, brassinosteroids and light-regulated development. Plant Cell Environ 20: 801-806.
Chun L, Kawakami A and Christopher DA (2001) Phytochrome A mediates blue light and UV-A dependent chloroplast gene transcription in green leaves. Plant Physiol. 125: 1957-1966.
De Lucas M, Davière J-M, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blazquez MA, Titarenko E, and Prat, S. (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451: 480-484.
Devlin PF, Halliday KJ, Harberd NP and Whitelam GC (1996) The rosette habit of Arabidopsis thaliana is dependent upon phytochrome action: novel phytochromes control internode elongation and flowering time. Plant J. 10: 1127-1134.
Devlin PF, Patel SR and Whitelam GC (1998) Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 10: 1479-1487.
Devlin PF, Robson PRH, Patel SR, Goosey L, Sharrock RA and Whitelam GC (1999) Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation and flowering time. Plant Physiol. 119: 909-915.
Devlin PF, Rood SB, Somer SE, Quail PH and GC Whitelam (1992) Photophysiology of the elongated internode (ein) mutant of Brassica rapa. The ein mutant lacks a detectable phytochrome B-like polypetide. Plant Physiol. 100: 1442-1447.
Franklin KA (2008) Shade avoidance. New Phytologist 179: 930-944.
Franklin KA (2009a) Light and temperature signal crosstalk in plant development. Current Opinion in Plant Biology 12: 63-68.
Franklin KA (2009b) Shade Avoidance. New Phytologist 179, 930-944.
Furuya M and Schäfer E (1996) Photoperception and signalling of induction reactions by different phytochromes. Trends Plant Sci. 1: 301-307.
Garner WW and Allard HA (1920) Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J. Agric. Res. 18: 553-606.
Gilbert IR, Jarvis PG and Smith H (2001) Proximity signal and shade avoidance differences between early and late successional trees. Nature 411: 792-795.
Halliday KJ, Koorneef M and Whitelam GC (1994) Phytochrome B and at least one other phytochrome mediate the accelerated flowering response of Arabidopsis thaliana L. to low red/far-red ratio. Plant Physiol. 104: 1311-1315.
Harberd NP (2003) Relieving DELLA restraint. Science 299: 1853-1854.
Harberd NP, Belfield E, and Yasumura Y (2009) The Angiosperm Gibberellin-GID1-DELLA Growth Regulatory Mechanism: How an "Inhibitor of an Inhibitor" Enables Flexible Response to Fluctuating Environments Plant Cell 21: 1328-1339.
Hennig L, Stoddart WM, Dieterle M, Whitelam GC and Schäfer E (2002) Phytochrome E controls light-induced germination of Arabidopsis. Plant Physiol. 128:194-200.
Heschel MS, Butler CM, Barua D, Chiang GCK, Wheeler A, Sharrock RA. Whitelam GC, Donohue K (2008) New roles of phytochromes during seed germination. International Journal of Plant Sciences 169: 531-540.
Hisamatsu T, King RW, Helliwell CA, Koshioka M (2005) The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiology, 138: 1106-1116.
Jackson SD, James P, Prat S and Thomas B (1998) Phytochrome B affects the levels of a graft transmissible signal involved in tuberisation. Plant Physiol. 117: 29-32.
Jackson SD and Prat S (1996) Control of tuberisation in potato by gibberellins and phytochrome B. Physiol. Plant. 81: 571-577.
Kasemir H (1983) Light control of chlorophyll accumulation in higher plants. In Encyclopedia of Plant Physiology, New series. Vol.16B, Eds Shropshire W and Mohr H, Springer-Verlag, Berlin. pp 662-686.
Keiller D and Smith H (1989) Control of carbon partitioning by light quality mediated by phytochrome. Plant Sci. 63: 25-29.
Koornneef M, Rolff E and Spruitt CJP (1980) Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana L. Heyhh. Z. Pflanzenphysiol. 100: 147-160.
Lagercrantz, U (2009) At the end of the day: a common molecular mechanism for photoperiod responses in plants? J. Exp. Bot. 60: 2501-2515.
Linkosalo, T., Lechowicz, M.J. (2006) Twilight far-red treatment advances leaf bud burst of silver birch (Betula pendula) Tree Physiology 26: 1249-1256.
López-Juez E, Nagatani A, Tomizawa K.-I, Deak M, Kern R, Kendrick RE and Furuya M (1992) The cucumber long hypocotyl mutant lacks a light-stable PHYB-like phytochrome. Plant Cell 4: 241-251.
Mathews S and Sharrock RA (1997) Phytochrome gene diversity. Plant Cell Environ 20: 666-671.
McCormac AC, Cherry JR, Hershey HP, Vierstra RD and Smith H (1991) Photoresponses of transgenic tobacco plants expressing an oat phytochrome gene. Planta 185: 162-170.
McCormac AC and Terry MJ (2002) Light-signalling pathways leading to the co-ordinated expression of HEMA1 and Lhcb during chloroplast development in Arabidopsis thaliana. Plant J 32: 549-560.
McCormac A, Wagner D, Boylan MT, Quail PH and Whitelam GC (1993) Photoresponses of transgenic Arabidopsis seedlings expressing introduced phytochrome B-encoding cDNA's:evidence that PHYA and PHYB have distinct photoregulatory functions. Plant J 4: 19-27.
McCormac A, Whitelam G and Smith H (1992) Light grown plants of transgenic tobacco expressing an introduced oat phytochrome A gene under the control of a constitutive viral promoter exhibit persistent growth inhibition by far-red light. Planta 188: 173-181.
Monte E, Al-Sady B, Leivar P, and Quail PH (2007) Out of the dark: How the PIFs are unmasking a dual temporal mechanism of phytochrome signaling Journal of Experimental Botany 58: 3125-3133.
Neff MM and Chory J (1998) Genetic interaction between phytochrome A, phytochrome B and cryptochrome 1 during Arabidopsis development. Plant Physiol 118: 27-36.
Parker MW, Hendricks SB, Borthwick HA and Scully NJ (1946) Action spectrum for the photoperiodic control of floral initiation of short-day plants. Bot Gaz 108: 1-26.
Reed JW, Nagatani A, Elich T, Fagan M and Chory J (1994) Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol 104: 1139-1149.
Reed JW, Nagpal P, Poole DS, Furuya M and Chory J (1993) Mutations in the gene for red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147-157.
Robson PRH, McCormac AC, Irvine AS and Smith H (1996) Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene. Nature Biotech. 14: 995-998.
Robson PRH, Whitelam GC and Smith H (1993) Selected components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiol. 102: 1179-1184.
Rodriguez-Falcon M, Bou J, and Prat S. (2006) Seasonal control of tuberization in potato: conserved elements with the flowering response. Annu. Rev. Plant Biology 57: 151-180.
Schmitt J (1997) Is photomorphogenic shade avoidance adaptive? Perspectives from population biology. Plant Cell Environ 20: 826-830.
Schmitt J, McCormac AC and Smith H (1995) A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome-mediated elongation responses to neighbours. American Naturalist 146: 937-953.
Selby CP, Sancar A (2006) A cryptochrome/photolyase class of enzymes with single-stranded DNA-specific photolyase activity Proceedings of the National Academy of Sciences, USA 103: 17696-17700.
Shinomura T, Nagatani A, Manzawa H, KubotaM, Watanabe M and Furuya M (1996) Action spectra for phytochrome A and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA 93: 8129-8133.
Smith H (1975) Phytochrome and Photomorphogenesis. McGraw-Hill. UK.
Smith, H (1982) Light quality, photoperception and plant strategy. Annu. Rev. Plant Physiol. 33: 481-518.
Smith H and Whitelam GC (1997) The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ 20: 840-844.
Smith H and Whitelam GC (1990) Phytochrome, a family of photoreceptors with multiple physiological roles. Plant Cell Environ 13: 695-707.
Somers DE, Sharrock RA, Tepperman, JM and Quail PH (1991) The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell 3:1263-1274.
Thomas B and Vince-Prue D (1997) Photoperiodism in Plants. Academic Press. London.
Weller JL, Perrotta G, Schreuder MEL, van Tuinen A, Koornneef M, Giuliano G and Kendrick RE (2001) Genetic dissection of blue-light sensing in tomato using mutants deficient in cryptochrome 1 and phytochromes A, B1 and B2. Plant J. 25: 427-440.
Whitelam GC and Smith H (1991) Retention of phytochrome-mediated shade avoidance responses in phytochrome-deficient mutants of Arabidopsis, cucumber and tomato. J. Plant Physiol. 139: 119-125.
Yanovsky MJ, Casal JJ and Whitelam GC (1995) Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 18: 788-794.
Yanovsky MJ and Kay SA (2002) Molecular basis of seasonal time measurement in Arabidopsis. Nature 419: 308-312.
08/19/09