PHOTOBIOLOGY of the CORNEA
Anthony P. Cullen
School of Optometry, University of Waterloo
Waterloo, Ontario, Canada N2L 3G1
acullen@uwaterloo.ca
Introduction
The cornea is the principal refracting (focusing) component of the eye in non-aquatic animals. Its physiology and anatomic structure are important for the maintenance of transparency in the visible spectrum. Light (visible radiation) has little direct effect on the cornea under normal circumstances.
Any form of radiant energy is potentially hazardous to the eye if it reaches and is absorbed by the tissues of the eye at levels capable of causing photochemical reactions, heat, structural changes or metabolic disturbance. This includes x-rays and other ionizing radiations, ultraviolet, visible and infrared, and millimetre and longer wavebands. The phototoxic effects of the optical wavebands of the electromagnetic spectrum may be due either to excessive radiant exposure or exposure in the presence of a photosensitizing agent. The effects may be biochemical or thermal.
In recent years it has become increasingly apparent that the effects of ultraviolet radiation (UVR) are more insidious and potentially detrimental to the eye and vision than had been suspected previously. The phototoxic effects of UVR on the cornea are initially photochemical. They may be acute, usually following a dose related latent period, long term as the sequelae of an acute exposure, or chronic following long term exposure to levels lower than those required to produce a clinically apparent acute response. Other ocular tissues that may be affected include the eyelids, conjunctiva, crystalline lens and retina. Infrared (IR) radiation interacts with corneal tissues to produce heat and a thermal response.
The structure and transmittance properties of the cornea play important roles in determining its response to exposure to a given wavelength of optical radiation. Knowledge of these is important in explaining and understanding some of the biological effects.
Corneal Structure
The cornea is the anteriorly projecting transparent part of the external coat of the eye. The tears bathe its external surface, and the aqueous humour, in the anterior chamber, the posterior surface. The crystalline lens, which provides secondary refractive functions, is located behind the iris.
Structurally the cornea (Figure 1) has five major zones, epithelium, Bowman layer (anterior limiting lamina), stroma (substantia propria), Descemet membrane (posterior limiting lamina), and endothelium. The epithelium is stratified with approximately five layers. The exposed cells are squamous and apoptotic. Beneath these the cells become more columnar through the intermediate ("wing") cells to the basal cells. Immediately below the epithelial basement membrane in human cornea is a layer of randomly oriented collagen fibrils designated Bowman layer. Approximately 90% of the corneal thickness consists of the stroma made up of regularly oriented bundles of collagen fibrils with a network of stromal cells. The posterior surface of the stroma is bounded by the endothelial basement membrane, Descemet membrane. The endothelium is a monolayer of non-mitotic mesothelial cells that lines Descemet membrane.
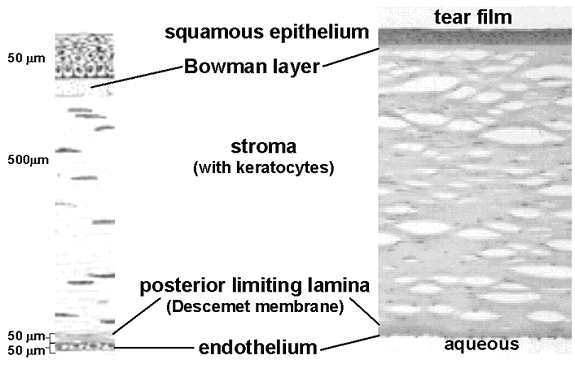
Figure 1. Anatomical zones of the human cornea. The top represents the anterior (external) surface of the cornea, which is bathed by the tear film, and the bottom is the posterior (aqueous) surface. The schematic on the left provides dimension and structural detail of the thin section light micrograph on the right.
Corneal Transmittance
The human cornea absorbs all UVR below ~280 nm (Figure 2). Above 280 nm there is a rapid increase in transmittance to 320 nm, and then a steady increase to a maximum in the visible spectrum (Ringvold, 1998). It is interesting to note that although the corneal epithelium comprises ~ 10% of corneal thickness, it accounts for most of the absorption of UVA (315-400nm). This is believed to result from the high protein and nucleic acid content of the epithelial cells.

Figure 2. Transmittance of the human cornea. The adult cornea absorbs all UVC radiation and commences transmittance at approximately 280 nm, and levels off at about 320 nm. There is little absorption from here through the near UVA, visible and infrared spectra, except for the water absorption bands in the infrared.
Corneal Phototoxic Response
The cornea is anatomically and physiologically a relatively simple structure, yet its responses to ultraviolet irradiation are quite varied. The acute response has been recognized since antiquity, and is known as photokeratitis. Occupational exposure to UVR has resulted in photokeratitis being given descriptors such as "snow blindness", "arc eye" and "welders flash". The primary response occurs in the epithelium, but the stromal cells (keratocytes) and endothelium can also be damaged. There appears to be no direct effects on the Bowman layer, the basement membranes, or the stromal fibrils.
In vivo Signs of Corneal Damage
The signs of ultraviolet damage are revealed by the use of a slit-lamp biomicroscope, which is arguably the most important diagnostic instrument used by optometrists and eye surgeons (Figure 3).

Figure 3. Corneal debris and granules. Direct illumination and specular reflection from the surface of a UV irradiated cornea. The oil on the tear film layer can be seen to the left. The glistening dots are cellular debris and the less defined deeper bluish patches are granules.
Evaluation of the pre-corneal tear film may reveal a discharge (produced by the conjunctiva) or debris. Debris appears as bright refractile dots in the tear film that represent both particles of disintegrated surface cells, and whole cells shed from the surface. This represents an acceleration of the physiological loss of surface cells. Exfoliation takes place by two mechanisms, shedding, where whole cells slough into the tear film, and apoptosis, in which cells disintegrate into the tear film. Ren and Wilson (1994) confirmed that epithelial shedding is accelerated by exposure to UVR. Suprathreshold radiant exposures result in full thickness loss of the stratified epithelium to the basement membrane. Exposed nerve fibre endings result in severe pain (Bergmanson, 1990).
Damage to either the corneal epithelium or endothelium disturbs the normal corneal deturgescence mechanisms (the means by which the cornea maintains optimal hydration and hence transparency). The stroma becomes edematous, and the elevated water content increases the corneal thickness (Cullen et al. 1984), and disrupts the structure of the stroma to produce increased scatter ("haze").
Granules are revealed by slit-lamp biomicroscopy by direct illumination, where they appear as tiny bluish amorphous opacities within the epithelial cell layer, or more dramatically by retro-illumination where their apparent color is determined by the reflected light from the fundus oculi. Their size corresponds to an epithelial cell or smaller and Duke-Elder (1972) considered them to represent swollen epithelial cells or fragmented nuclei. Later ultrastructural studies showed autolysis of the wing cells with secondary lysosomal vacuoles, and normal cell nuclei (Cullen, 1980). It appears that membranes of intracellular organelles are particularly susceptible to UVR damage.
The presence of flare (Figure 4) in the anterior chamber represents the release of plasma proteins into the aqueous humour from the blood vessels of the anterior uvea (the iris and ciliary body). A conical beam of light is scattered (Tyndall effect) by the small particles. Normally the anterior chamber is optically empty. Flare occurs due to inflammatory disease of the anterior uvea or following trauma to the cornea.
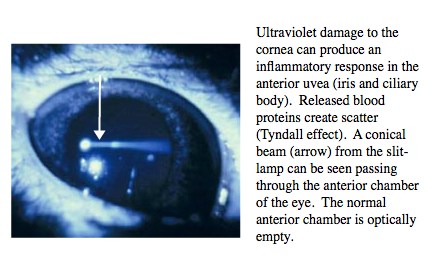
Figure 4. Ultraviolet damage to the cornea can produce an inflammitory response in the anterior uvea.
Ultraviolet Action Spectra
The threshold radiant exposure that produces a defined response at a given wavelength when plotted over a given waveband provides the action spectrum for the waveband. The action spectra for damage to the cornea and other ocular tissues are presented in Figure 5. The spectra for humans, monkeys and rabbits show close proximity, with maximum sensitivity at approximately 270 nm. Also, the spectra derived from coherent (laser) UV sources (Zuchlich, 1998) show close agreement with those derived from incoherent (arc) sources.
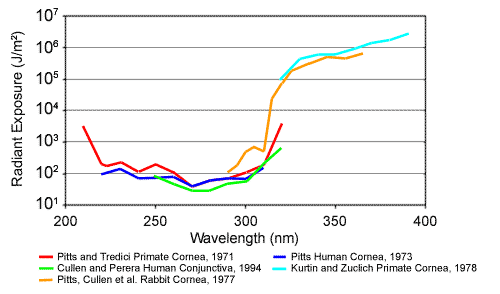
Figure 5. Ultraviolet action spectra. (Cullen, 2002). These are spectra of for threshold ultraviolet damage to the corneal epithelium for different species as derived by different researchers. Note the similarity and overlap of the curves. The green curve is for damage to human conjunctival epithelium. UV-A is far less hazardous to the cornea than UV-B and UV-C. (Figure created by Ms. Erin Chaney, US Army Center for Health Promotion and Preventive Medicine. Aberdeen Proving Ground, MD 21010-5433 USA. Edited for use in the PSO.)
Symptoms of Photokeratitis
The symptoms of photokeratitis develop several hours after exposure, with the latent period inversely proportional to the radiant exposure. Superficial corneal epithelial damage produces a gritty feeling in the eye, coupled with photophobia and tearing. Any corneal oedema results in haze or clouding, and a deterioration of vision. Higher exposures resulting in epithelial exfoliation produce excruciating pain and blepharospasm (involuntary closure of the eyelids). Fortunately the cornea re-establishes itself rapidly, and the symptoms usually abate within two days, leaving no clinically detectable signs.
A feature of keratitis, reported by trained visual psychophysical observers, is that following exposure and prior to the onset of symptoms, vision becomes clearer. The loss of epithelial cells and their related scatter serves to enhance the contrast sensitivity of the retina.
Corneal Response to Chronic Exposure to UVR
Chronic exposure to UVR contributes to a number of degenerative conditions of the surface of the cornea. Climatic droplet keratopathy (Figure 6) produces severe visual impairment by depositing material in the superficial stroma and Bowman layer. It is postulated that UVR exposure may photochemically alter diffusible plasma proteins reaching the cornea. Epidemiological studies in Labrador (Johnson, 1981) and on the Chesapeake Bay (Maryland) (Taylor et al., 1989) have confirmed the relationship between total broadband UV exposure and the prevalence of droplet keratopathy. Reflected UV, from snow, water or white sand plays a greater role than direct sunlight. This is because the brow, eyelids and lashes provide protection from overhead rays.
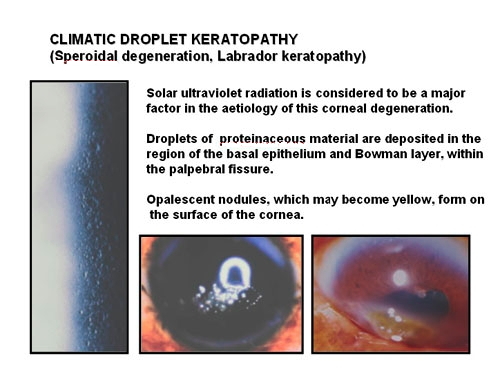
Figure 6. Climatic droplet keratopathy. The left image is a is a high magnification slit-lamp photograph of early droplet keratopathy revealing the small droplets in the corneal epithelium and Bowman layer. The two lower photographs illustrate the nodular and yellowing stages, respectively.
Pterygium (Figure 7) is a wing shaped sheet of fibrovascular tissue that invades the cornea. Ambient UVR due to sunlight is considered the most significant environmental causative factor. Pterygia are more common in sunny climates and at high altitudes. Studies (Moran and Hollows, 1984; Taylor et al., 1989) have demonstrated a cumulative dose relationship between UVR and pterygium. The role of solar UVR in the developmenrt of pterygium has been emphasized by results from different populations and environments. Comparison of the numbers of cases of pterygium (and also cataract) in four indigenous populations in the Amazonian rainforest revealed that differences in the amount of sun exposure are largely responsible for the observed differences in the prevalence of these two diseases (Paula et al., 2006). A study in a Croatian island population found a prevalence of pterygium of 23% amongst farmers and fishermen, compared with none in subjects residing in urban areas (Vojnikovic et al., 2007). In a Tibetan population living at high altitude in China, those individuals who seldom used sunglasses had a five-fold increase in the risk of pterygium compared to the rest of the population (Lu et al., 2007). Similarly, workers who have been exposed to industrial UV (which often included UV-C, < 280nm) have a higher prevalence of pterygia (Karai et al.,1984). Recurrence of pterygium following surgery has been found to be reduced in those exposed to less sunlight (Sekeli et al., 2007).
The predominantly nasal location of pterygia has been explained on the basis of peripheral light focussing. UVR incident horizontally from behind the coronal plane is focused onto the medial anterior chamber angle (Coroneo, 1990; Kwok and Coroneo, 1994) beneath the location of the limbal corneal stem cells. As stem cells are actively dividing they are likely to have a lower damage threshold than non-mitotic corneal epithelial cells.




Figure 7. Pterygium: Primary and Recurrence. Note that the top graphic (Primary) shows that the right edge of the cornea and iris are obscured by the overgrowth of fibrovascular tissue. The lower graphic (Recurrence) shows considerably more overgrowth of the cornea, and extensive conjunctival scarring.
Corneal Endothelium
The corneal endothelium is considered non-mitotic in the normal primate eye. Thus, if cells are lost due to ageing, trauma, inflammation or surgery, the remaining cells enlarge to maintain coverage of the Descemet membrane, with the corresponding reduced cell count. In the ageing eye, we can also record varying sizes of endothelial cells (polymegathism), and many non-hexagonal cells (pleomorphism). The surface of the endothelium may become protuberant locally due to the formation of small corneal guttae (nodules) in Descemet membrane, decribed as cornea guttata. Some eyes may develop more serious forms of endothelial dystrophy, for example Fuchs dystrophy.
We have shown using pachometric and ultrastructural methods (Cullen et al. 1984; Doughty et al. 1990) that UV-B is capable of damaging endothelial structure and function. The endothelial ultraviolet damage threshold (Hendo) is approximately 0.125 J/cm2 (at the anterior corneal surface) and may be derived from the epithelial ultraviolet damage threshold (Hepi) by the following expression
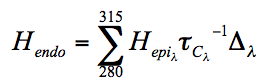
where
 and
and  are corneal transmittance and epithelial threshold at wavelength
are corneal transmittance and epithelial threshold at wavelength  and
and  is the wavelength interval.
is the wavelength interval.
It is possible that UVR, either acute or chronic exposure, may play a role in the ageing processes of the endothlium as it does in other tissues.
Conjunctival Phototoxic Response
The conjunctiva is a thin transparent mucous membrane that covers the sclera of the anterior globe, and reflects anteriorly to line the eyelids. The non-keratinized epithelium of the bulbar conjunctiva is continuous with that of the cornea, and shares characteristics such as elongated basal cells and flattened surface cells. The interpalpebral zones are exposed to the environment, and using irradiance levels of the same order of magnitude as solar UVB the action spectrum for acute photoconjunctivitis (Figure 5) approximates that for human photokeratitis, but with slightly lower thresholds (Cullen and Perera, 1994). Photoconjunctivitis is painless at threshold exposure levels, and follows a similar time course to photokeratitis. Clinically, its presence is determined by injected conjunctival blood vessels, oedema (chemosis) and damaged epithelial cells, which stain with rose bengal.
Chronic exposure to UV, specifically solar exposure, has been implicated in several diseases that involve the interpalpebral conjunctiva, including spheroidal degeneration, pinguecula, pterygium, hyperkeratosis, carcinoma-in-situ, and squamous cell carcinoma. Histopathological studies (Clear et al. 1979) have demonstrated that actinic keratosis, pterygium and pinguecula present a continuous spectrum of the same pathological processes and etiology, with the potential end point of conjunctival squamous cell carcinoma. Immunological (Pinkerton et al. 1984) and chronic irritative (Hill and Maske, 1989) mechanisms have been proposed.
Phototoxins and the Cornea
An effective photoxin absorbs photons efficiently, is excitable to a triplet state, donates or accepts electrons readily and/or generates singlet oxygen and other reactive species. In order to produce corneal effects, the phototoxin must be absorbed by or be in contact with corneal tissue(s), and be reached by the exciting wavelengths (see the Basic Photosensitization module). There are several potential routes by which endogenous or exogenous phototoxins may reach the cornea (Figure 8). The tears contain secretions from the lacrimal gland and accessory glands (mainly water), the tarsal (Meibomian) glands (oil), and the conjunctival goblet cells (mucin). In addition, airborne toxins, either water or lipid soluble, may come into contact with the corneal epithelium. The endothelium is nourished by the aqeous humor, which is mainly secreted by the ciliary body but does include some passive secretions. The normal cornea is free from blood and lymph vessels except for capillary loops around the circumference, derived from the vessels of the conjunctiva and episclera.
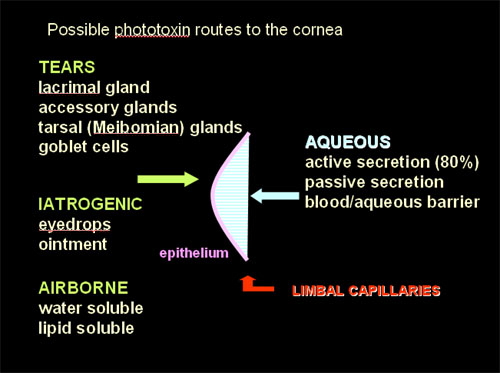
Figure 8. Possible routes by which phototoxins could enter the corneal tissues (Cullen, 2002). Five routes of access are suggested and depicted, 2 from the environment (exogenous), and 3 from within the organism (endogenous).
Exogenous photosensitizers reach the ocular tissues directly or indirectly via the circulation when they are administered systemically for the management of diseases. The number of drugs with suitable molecular structure is high, but few have been studied specifically for their phototoxic effects on the eye. Notable exceptions are the psoralens and the tricyclics. The former have been implicated in cataracts in experimental animals, and the latter in deposition of photoproducts in the cornea and crystalline lens. The list of potential ocular phototoxins includes the sufonamides, sulfonylureas, chlorothiazides, pheothyazides, and non-steroidal anti-inflammatory drugs. Because some of these agents have other ocular side effects, it has been proposed that the side effects of these drugs are due to their photoxic abilties. The task of relating any effect to a photosensitizing mechanism continues to be difficult (Dayhaw-Barker and Barker, 1986).
Potential phototoxic effects on the cornea may become real phototoxic effects if the threshold for damage (increased sensitivity) is lowered, or if the action spectrum extends into the UV-A and visible spectral bands. Those compounds that are deposited in the corneal epithelium are lost due to migration of cells from the basal layer to the surface where they slough off. Theoretically the lifespan of an epithelial cell is about seven days. There have been relatively few reports (Nishida et al., 1992) of phototoxic effects involving the cornea. This could be because the effects on the lens and retina can produce more severe vision impairment. Nonetheless, a century ago it was recognized that workers exposed to the volatiles produced by coal-tar pitch and sunlight developed a phototoxic keratoconjunctivitis. The use of ophthalmic ointments containing aciflavine was observed to cause severe keratitis in Brazil (Duke-Elder, 1972).
The paucity of documented phototoxic effects on the cornea despite the presence of considerable numbers of known endogenous and exogenous photosensitizing agents suggests that the human cornea is much less susceptible to their influence than the skin. This could be due to the unique structure and physiology of the cornea or its sophisticated defense mechanisms.
Protective Strategies
The major natural phototoxic hazard to the cornea and conjunctiva is UV-B present in sunlight. The levels of UV-B reaching the eye are increased at high altitude and by reflection. Fresh snow is an excellent reflector of ultraviolet radiation. The public health need for eye protection from ambient and industrial UVR has been emphasized (Sliney, 2001).
The eye is located in the bony orbit, and the structure of the skull and ocular adnexa (the brow, eyebrows, lids and eyelashes) provide considerable protection from overhead solar irradiance (Sliney, 1999) but not from lateral and reflected ultraviolet. Human tears, which are essentially water, are transparent to the entire optical waveband, except for negligible absorption of infrared. They give no protection against UVR. Theoretically, topical drugs that stabilize membranes could potentially offer some protection for the cornea. These include corticosteroids, which would be contraindicated for a variety of reasons, including the development of ocular complications. Other eye drops, such as 8-hydroxy-1-methylchinlinium methysulphate, have been found to be of little use in protecting the eye from solar UVR (Daxer, 1998).
The normal eye has some defences against oxidative and photo-induced damage, including antioxidant enzymes such as superoxide dismutase and catylase, and antioxidants such as vitamins C and E, lutein, zeaxanthin, lycopene, glutathione and melanin. Most of these begin to decrease after 40 years-of-age, resulting in less protection from optical radiation induced damage to various structures of the eye (Roberts, 2002). The use of dietary supplements containing antioxidants, free radical scavengers and trace minerals have not been found effective in the management of UVR related corneal diseases.
The use of appropriate ophthalmic and industrial absorptive glass and plastic materials to protect ocular tissues from excessive exposure to UVR is generally accepted and well understood. Most modern spectacle lenses give significant protection from axially incident UVR but neither these nor regular sunglasses offer adequate protection from peripherally incident or reflected rays. It has been estimated that wearing both regular sunglasses and a hat gives essentially no protection for the infra-nasal crystalline lens, and may even increase exposure due to the reduction in squinting. Ski-goggles and wrap-around sunglasses provide the best total protection for the eye.
UV absorbing soft contact lenses that cover the limbal region provide protection to the internal structures of the eye at least equivalent to wrap-around sunglasses or goggles. The interpalpebral conjunctiva and lids remain unprotected with soft lenses and the peripheral cornea and internal structures have less protection with rigid lenses. Most contact lenses are transparent in the visible spectrum and do not protect against the effects of high radiant exposures to or the glare produced by this waveband. Thus UV absorbing contact lenses are not suitable as a replacement for traditional forms of protection in leisure or work environments, but they do offer additional protection in situations where it is not feasible to wear sunglasses or goggles (e.g. some watersports). Wearing UV absorbing contact lenses does not negate the efficacy of other protective strategies, and for habitual contact lens wearers the addition of UV absorption provides a further level of safety, whether or not traditional protective approaches are used (Cullen,2005).
References
Bergmanson JP. 1990. Corneal damage in photokeratitis - why is it so painful. Optom. Vis. Sci. 67:407-13.
Clear AS., Chirambu MC, Hutt MSR, 1979. Solar keratosis, pterygium and squamous cell carcinoma of the conjunctiva in Malawi. Br. J. Ophthalmol. 63(2): 102-1909.
Coroneo MT.1990. Albedo concentration in the anterior eye: a phenomenon that locates some solar diseases. Ophthalmic Surg 1990;21:60-6.
Cullen AP. 1980. Ultraviolet Induced Lysomal Activity in the Corneal Epithelium, v. Graefes. Achiv. Ophtha. 214: 107-118.
Cullen AP, Chou BR, Hall MG, Jany SE. 1984. UV-B Damages Corneal Endothelium. Amer. J. Optom. Physio.l Opt. 61(7): 473-478.
Cullen AP, Perera SC, 1994. Sunlight and human conjunctival action spectrum. In Ultraviolet Radiation Hazards. eds. Sliney D.H. and M. Belkin. SPIE Proceedings 2134B, 24-30.
Cullen AP. 2002. Photokeratitis and other phototoxic effects on the cornea and conjunctiva. Internat. J. Toxicol. 21(6):455-464.
Cullen AP. 2005. Contact lenses and the ophthalmohelioses. Rev. Optom. Jan. Suppl. 17-23.
Daxer A, Blumthaler M, Schreder J, Ettl A. 1998. Effectiveness of eye drops protective against ultraviolet radiation. Ophthalmic. Res. 30:286-90.
Dayhaw-Barker P, Barker FM. 1986. Photoeffects on the Eye. In Photobiology of the Skin and Eye. ed. E.M. Jackson, 117-149, New York: Marcel Dekker, Inc.
Doughty MJ, Cullen AP. 1990. Long-term effects of a single dose of ultraviolet-B irradiation on albino rabbit cornea - II Deturgescence and fluid pump assessed in vitro. Photochem. Photobiol. 54(4): 439-449.
Duke-Elder S. 1972. System of Ophthalmology, Vol. XIV, Part 2, Non-mechanical Injuries, p.926, London: Henry Kimpton
Hill JC, Maske R. 1989. Pathogenesis of pterygium. Eye 3(2): 218-226.
Johnson GJ. 1981.Aetiology of spheroidal degeneration of the cornea in Labrador, Br. J. Ophthalmol. 65:270-283
Karai I, Horiguchi S. 1984. Pterygium and ultraviolet radiation: a positive correlation. Br. J. Ophthalmol. 68: 343-346.
Kurtin WE, Zuchlich JA, 1978. Action spectrum of oxygen-dependent near-ultraviolet induced corneal damage. Photochem. Photobiol. 27: 329-333.
Kwok LS, Coroneo MT. 1994. A model for pterygium formation. Cornea 13:219,24.
Lu P, Chen X, Kang Y, Ke L, Wei X, Zhang W. 2007. Pterygium in Tibetans: a population-based study in China, Clin. Experiment. Ophthalmol., 35:828-33.
Mora DJ, Hollows FC. 1985. Pterygium and ultraviolet radiation: a positive correlation. Br. J. Ophthalmol. 68: 343-346.
Nishida K, Ohashi Y, Kinoshita S, Kiritoshi A, Manabe R. 1992. Endothelial decompensation in a schizophrenic patient receiving long-term treatment with tranquilizers. Cornea. 11(5):475-478.
Paula JS, Thorn F, Cruz AA. 2006. Prevalence of pterygium and cataract in indigenous populations of the Brazilian Amazon rain forest, Eye, 20:533-536.
Pinkerton OD, Hokama Y, Shigemura LA. 1984. Immunologic basis for the pathogenesis of pterygium. Am. J. Ophthalmol. 98(8): 225-228.
Pitts DG, Tredici TJ. 1971. The effects of ultraviolet radiation on the eye. Amer. Indust. Hyg. Assoc. J. 32:235-246.
Pitts DG. 1973. The ultraviolet action spectrun and protection criteria. Health Physics. 25: 559-566.
Pitts DG, Cullen AP, Dayhaw Hacker P. 1977. Ocular effects of ultraviolet radiation from 295 to 265 nm. Invest. Ophthalmol. Vis Sci. 16: 932-939/
Ren H, Wilson G. 1994. The effect of ultraviolet-B irradiation on the cell shedding rate of the corneal epithelium. Acta. Ophthalmologica. 72: 447-452
Ringvold A. 1998. Corneal epithelium and UV-protection of the eye. Acta Ophthalmol. Scand. 76: 149-153
Roberts JE. 2002. Screening for ocular phototoxicity. Int. J. Toxicol. 21:491-500
Sekelj S, Dekaris I, Kondza-Krstonijevic E, Gabric N, Predovic J, Mitrovic S. 2007. Ultraviolet light and pterygium, Coll. Antropol., 31 Suppl 1:45-47.
Sliney DH. 1999. Geometrical assessment of ocular exposure to environmental UV radiation - implications for ophthalmic epidemiology. J. Epidemiol. 9:S22-S32
Sliney DH.2001. Photoprotection of the eye - UV radiation and sunglasses. J. Photochem. Photobiol. B, 64:166-75.
Taylor HR, West SK, Rosenthal FS, Munoz B, Newland HS, Emmett EA. 1989, Corneal changes associated with chronic UV irritation. Arch. Ophthalmol. 107:1481-1484.
Vojnikovic B, Njiri S, Coklo M, Toth I, Spanjol J, Marinovic M. 2007. Sunlight andincidence of pterygium on Croatian Island Rab-epidemiological study, Coll. Antropol., 31 Suppl 1:61-62.
Zuchlich JA. 1998. The Cornea - Ultraviolet Action Spectrum for Photokeratitis. In Measurement of Optical Radiation Hazards, ed. D. Sliney, 143-160, Oberschleissheim: International Commission for Non-Ionizing Radiation Protection.
01/24/09
09/22/11