Thomas M. Brennan
Department of Biology
Dickinson College, Carlisle, PA 17013
brennant@dickinson.edu
Introduction
The functioning of the Earth's ecosystem is dependent upon a continuous input of light energy in the form of photons coming from the sun. The process by which light energy is captured and incorporated into molecules that can be used by living organisms is called photosynthesis. It takes place in green plants, algae, and some kinds of bacteria. Organisms can be grouped into two major categories: autotrophs, which carry on photosynthesis and manufacture their own food, and heterotrophs, which do not perform photosynthesis themselves but must consume autotrophs or other organisms that ate autotrophs. In other words, photosynthetic organisms are at the base of the food chain, and with only a very few exceptions all organisms on Earth depend upon photosynthesis as the source of their food and energy.
Although photosynthesis is a very complex process, it can be divided into two parts. In the first part, light energy is captured and used to make high energy chemical molecules. In the second part, those high energy molecules are used to convert atmospheric carbon dioxide (CO2) into carbohydrates and other energy containing compounds. These then serve as food for the photosynthetic plant itself or for an animal that eats the plant. During this process, water molecules (H2O) are split apart and the oxygen from them is released into the atmosphere as oxygen gas (O2). This is an important byproduct of photosynthesis because most organisms have evolved ways of using O2 to help them process their foods more efficiently. Some of the O2 also moves into the upper atmosphere where it forms ozone (O3), which is important in protecting the Earth's surface from ultraviolet radiation.
We will now examine these and other aspects of photosynthesis in more detail.
Capture of Light Energy and Formation of High Energy Molecules
This first part of photosynthesis is sometimes referred to simply as the "light reactions." In essence it is an energy conversion process, i.e. the conversion of light energy into chemical energy. This discussion will be based upon the way the process is believed to function in higher plants, although the fundamental mechanisms are generally similar in other types of organisms.
Photosynthetic Pigments and the Structure of the Leaf
The first law of photochemistry and photobiology tells us that in order for light to have an effect in a biological system it must first be absorbed. Pigments are molecules that are specialized for the absorption of light, and they therefore must be found in all photosynthetic organisms. Two major classes of photosynthetic pigments that occur in higher plants are the chlorophylls and the carotenoids.
Chlorophylls consist of a light-absorbing porphyrin ring with a magnesium atom at the center and a long phytol tail that anchors the molecule in a membrane (Figure 1). They absorb light in the blue and red parts of the spectrum, but the green wavelengths are transmitted or reflected. This is what makes leaves appear green in color. Chlorophylls a and b both occur in higher plants. They have slight differences in structure and absorbance spectra. Protochlorophyll is a biosynthetic precursor of chlorophyll.
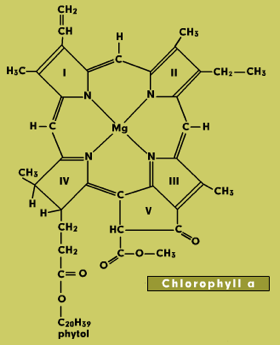
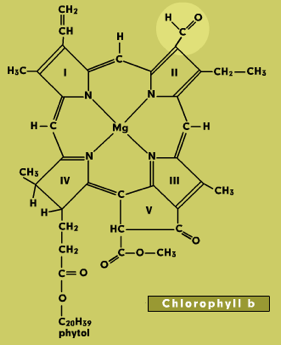
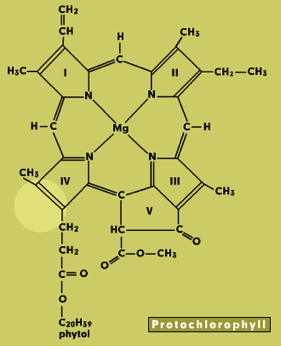
Figure 1. Chlorophyll Structures. The structural differences from chlorophyll a are spotlighted for each selection.
Carotenoids (Figure 2) are yellow to orange in color. They extend the range of wavelengths that can be utilized in photosynthesis by absorbing some light in the blue region of the spectrum that chlorophyll does not absorb. They also participate in photosynthesis by protecting the chlorophyll from reactive oxygen compounds. Carotenoids are responsible for the brilliant colors of autumn leaves. They are present in leaves all the time, but remain unseen until the larger quantities of chlorophyll begin to break down with the onset of short days and cool weather.
The carotenoids and other non-chlorophyll pigments that participate in photosynthesis are referred to as accessory pigments. Their structures and properties vary among different species (Figure 2).
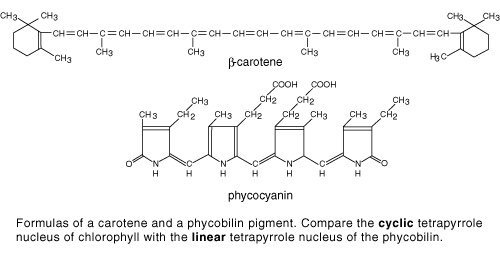
Figure 2. Accessory pigments. Two examples of pigments that may assist chlorophyll in photosynthetic light absorption.
The photosynthetic pigments are contained in the membranes of subcellular organelles called chloroplasts (Figure 3), which reside inside leaf cells. The interior air spaces of the leaf are connected to the outside atmosphere through tiny pores called stomata, which allow for the inward movement of CO2 and the outward movement of O2. The double membrane surrounding the chloroplast is permeable to small uncharged molecules such as CO2 and O2, thereby allowing for exchange of these gases during photosynthesis.
The pigment-containing membranes inside the chloroplast are referred to as thylakoids, and are arranged in stacks called grana, which are strikingly similar to the arrangement of disks in the photoreceptor cells of the vertebrate retina. This arrangement of stacked membranes has apparently evolved independently several times as an extremely efficient system for the interception and capture of light energy. The chlorophyll and carotenoid molecules in the thylakoid membranes are not free, but exist in the form of pigment-protein complexes, sometimes referred to as "light-harvesting complexes." These proteins maintain the pigments in the proper orientation for efficient energy transfer, and also bring about slight changes in the wavelengths of light they can absorb.
The non-membranous region inside the chloroplast is called the stroma. It contains an aqueous solution of enzymes and various metabolites that participate in the reduction of CO2 to carbohydrate via the Calvin Cycle, as described below.
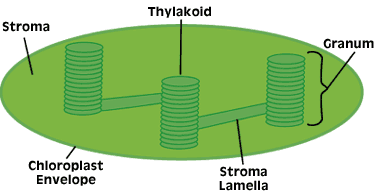
Figure 3. The chloroplast. Photosynthesis occurs in the chloroplasts of algae and higher plants. Capture of light energy takes place on the interior membrane surfaces, while reduction of CO2 to carbohydrate occurs in the stroma.
Absorption of Photons and Transfer of Energy
When a plant is exposed to light, photons of appropriate wavelength will strike and be absorbed by the pigment-protein complexes arrayed on the thylakoid membranes. When this happens, the energy of the photon is transferred to the pigment molecule, thus causing the pigment to go into an electronically excited state. In other words, the energy originally associated with the photon is now contained within the pigment molecule. Once a pigment molecule has gone into an excited state, it is possible for the excitation energy to be transferred to an adjacent pigment molecule.
Dispersed among the pigment-protein complexes on the thylakoid membranes are special structures called reaction centers. They are fewer in number than the light-harvesting complexes, and consist of proteins, chlorophyll, and several other pigments in a highly structured arrangement. This will be described later in a detailed module. There are two types of reaction centers, called Photosystem I (PSI or P700) and Photosystem II (PSII or P680).
The array of pigment-protein complexes on the thylakoid membranes functions as an antenna for gathering light energy. Excitation energy is transferred from one chlorophyll to another until the energy reaches a reaction center (Figure 4). This causes one of the chlorophyll molecules within the reaction center to go into an excited state.
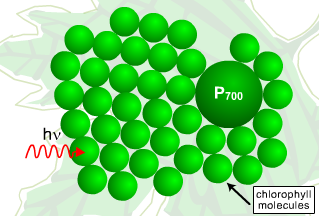
Figure 4. An Antenna Complex. The array of pigment-protein molecules (e.g., chlorophyll) that absorbs light is referred to as an antenna complex. In this simplified figure, energy is absorbed from a photon of light, and transferred stepwise to the reaction center, P700. In the reaction center, the energy is imparted to an electron, which is passed on to a chain of carriers.
Formation of High Energy Chemical Compounds
Two kinds of energy-containing molecules are formed in the light reactions of photosynthesis: adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide phosphate (NADPH), which serves as a source of reducing power.
The PSI and PSII reaction centers are interconnected by a series of compounds that function as electron carriers (Figure 5). An excited electron in a PSII reaction center enters this chain of carriers and moves from one to the next until it reaches a PSI reaction center. This transport of an electron between the two types of reaction centers results in the pumping of hydrogen ions (H+) across the thylakoid membrane, thus forming a gradient with a high H+ concentration inside the thylakoid compartments and a relatively low concentration on the stroma side. The potential energy associated with this gradient is then used to form ATP by a mechanism similar to that by which ATP is generated in mitochondria.
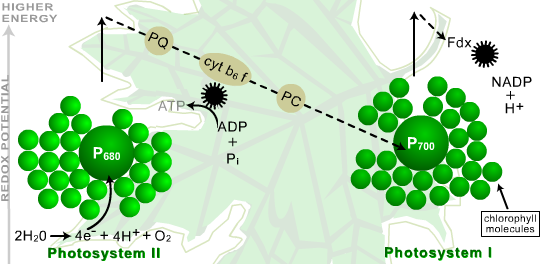
Figure 5. Photosystems I and II. The relationship between PSI and PSII, the connecting carrier chain and the generation of high energy compounds is shown as an electron passes through the system, gaining and losing energy. P680 = reaction center of PSII; P700 = reaction center of PSI; PQ = plastoquinone; cytb6f = cytochrome c b6f; PC = plastocyanin; Fdx = ferredoxin.
The next step in the process is that an excited electron in PSI is transferred to a molecule of NADP, along with an H+, thereby reducing it to NADPH. Notice that the electrons leaving PSI are being replaced by ones that came through the electron carriers from PSII.
Finally, in order for this process to continue, the electrons that were removed from PSII have to be replaced. This is achieved by the splitting of an H2O molecule, which yields electrons, H+, and oxygen. The oxygen from two water molecules forms O2, which then passes through the stomata into the atmosphere.
In summary, what has been achieved is the conversion of photon energy into chemical energy in the form of ATP and NADPH, with the concomitant formation of O2.
Reduction of Atmospheric CO2 to Carbohydrate
The second major phase of photosynthesis involves the conversion of CO2 from the atmosphere into carbohydrates and other biological molecules. This set of reactions is sometimes referred to as the "dark reactions" of photosynthesis because light is not directly involved. However, this terminology is somewhat misleading because light is required for the formation of the ATP and NADPH needed for energy and reducing power. If a photosynthesizing plant was suddenly put in the dark, the so-called dark reactions would only continue until the supply of ATP and NADPH was depleted.
The conversion of CO2 into carbohydrate takes place in the chloroplast stroma and follows a complex metabolic pathway called the Calvin Cycle or the Reductive Pentose Phosphate Pathway, each step of which is catalyzed by a specific enzyme. The details of this pathway will be dealt with in another module, but its overall form is shown in Figure 6. Carbon dioxide first combines with a five carbon compound called ribulose-1,5 -bisphosphate (RUBP), which then immediately splits into two three carbon compounds. Using energy and reducing power from ATP and NADPH, these are converted into 3-phosphoglyceraldehyde (3PGAL), which can be used by the plant to manufacture carbohydrates and various other biological molecules and to regenerate RUBP.
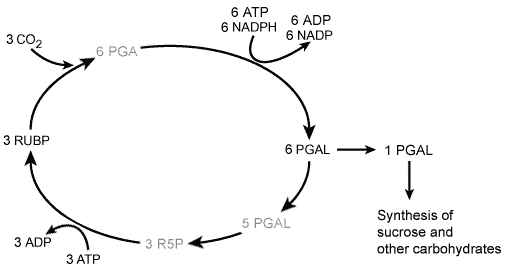
Figure 6. The Calvin Cycle. In this series of reactions, only a few of which are shown here, carbon dioxide from the atmosphere is converted into carbohydrates. Understanding this cycle earned a Nobel Prize for Melvin Calvin in 1961.
In order for the Calvin Cycle to continue, there must be a continuous supply of RUBP. Thus not all of the 3PGAL produced can go into the synthesis of carbohydrates - a significant portion (about 5 molecules out of 6) must be used to make fresh RUBP. This also requires ATP coming from the light reactions, and is what gives the pathway its cyclic nature.
In summary, ATP and NADPH formed in the light reactions of photosynthesis are used to convert atmospheric CO2 into carbohydrates, which represent a stable, long term form of energy storage. Overall, energy that originated as photons from the sun is now contained in carbohydrates and other molecules that serve as food for the plant or for an animal that eats the plant.
Environmental Aspects of Photosynthesis
Because of the fundamental biological significance of photosynthesis and its importance in agricultural productivity, a considerable amount of research has been directed toward understanding the effects that environmental factors such as light, temperature, rainfall, salinity, and disease have on the process. In recent years, the effects of elevated atmospheric CO2 and of increased ultraviolet radiation exposure due to depletion of the Earth's ozone layer have received considerable attention. The potential for forests and other photosynthetic ecosystems to mitigate increasing atmospheric CO2 is also an active area of research.
Different species, and in some cases even different cultivars of the same species, vary widely in their ability to tolerate the multitude of environmental stresses that a photosynthesizing plant may encounter. The natural geographic distribution of plant species can be understood in terms of their ability to photosynthesize efficiently under the range of conditions that they encounter in the Earth's various ecosystems. Likewise, environmental factors govern the range over which specific agricultural crops can be successfully introduced and cultivated.
Both traditional plant breeding methods and the techniques of modern molecular biology are being used to produce plants that can maintain their vigor and sustain high rates of photosynthesis when subjected to environmental stress. These approaches have been very successful in the past and promise to further increase crop productivity and decrease the need for fertilizers, herbicides, and insecticides. These topics will be dealt with in more detail in another module.
Suggested Readings
Blankenship, R. E. 2002. Molecular Mechanisms of Photosynthesis. Blackwell Science Publishers, Oxford, UK, Malden, MA, USA.
Jansson, J. 1994. The Light-Harvesting Chlorophyll a/b-Binding Proteins. Biochimica Biophysica Acta, 1184: 1919.
Long, S. P., E. A. Ainsworth, A. Rogers, and D. R. Ort. 2004. Rising Atmospheric Carbon Dioxide: Plants FACE the Future. Annual Review of Plant Biology, 55: 591-628.
Nelson, N. and C. F. Yocum. 2006. Structure and Function of Photosystems I and II. Annual Review of Plant Biology, 57: 521-565.
Raghavendra, A. S., ed. 1998. Photosynthesis: A Comprehensive Treatise. Cambridge University Press, Cambridge, UK, New York, USA.
Raven, P. H., G. B. Johnson, J. Losos, and S. Singer. 2005. Biology, 7th. ed. McGraw-Hill, New York.
Szalai, V. A. and G. W. Brudvig. 1998. How Plants Produce Dioxygen. American Scientist, 86: 542-551.
Taiz, L. and E. Zeiger. 2006. Plant Physiology, 4th. ed. Sinauer Associates, Sunderland, MA.
Wild, A. and R. Ball. 1997. Photosynthetic Unit and Photosystems: History of Research and Current View. Backhuys Publishers, Leiden.
3/25/08